10
A clash of appetites: food-related dimensions of human–animal conflict and disease emergence
A clash of appetites
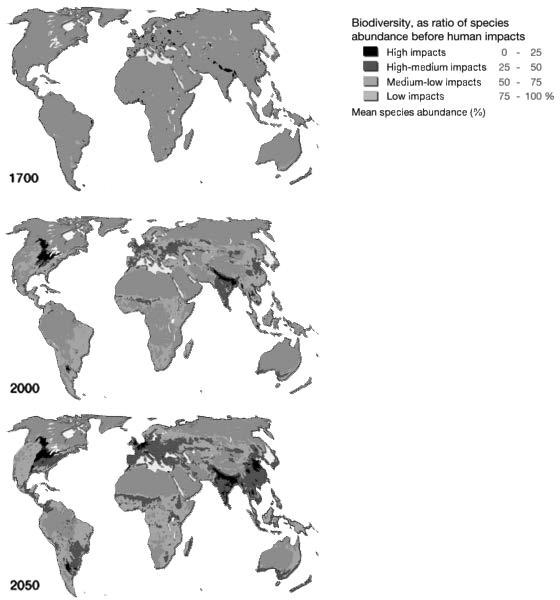
In 2011 the human population crossed the 7 billion threshold, more than doubling the global population just 50 years earlier, leading to radical changes in human food production systems (Food and Agriculture Organization Corporate Statistical Database [FAOSTAT] 2014; United Nations Population Fund 2011). While 66 per cent of people lived rurally in 1961, more than half now live in cities (FAOSTAT 2014). Land clearing for human food production – including growing crops for human consumption or production of livestock/fish feed, as well as grazing livestock – has converted around 40 per cent of the earth’s surface to cropland and pasture (Foley et al. 2005) (Figure 10.1a). Demand is largely met with increases in production per land area, as a result of technological advances (e.g. fertiliser and irrigation), improvements in plant and livestock genetics, and intensification of livestock industries. Taken together, humans and livestock now make up approximately 96 per cent of global mammal biomass (Bar-On et al. 2018). Simultaneous with increasing population and food production, rising per capita incomes in recent decades have changed human diets. The average human now consumes 2,870 kilocalories (kcal) per person per day, up from 2,196 kcal per person per day in 1961, with a larger proportion of the diet comprising food of domestic animal origin (FAOSTAT 2014; Kearney 2010).
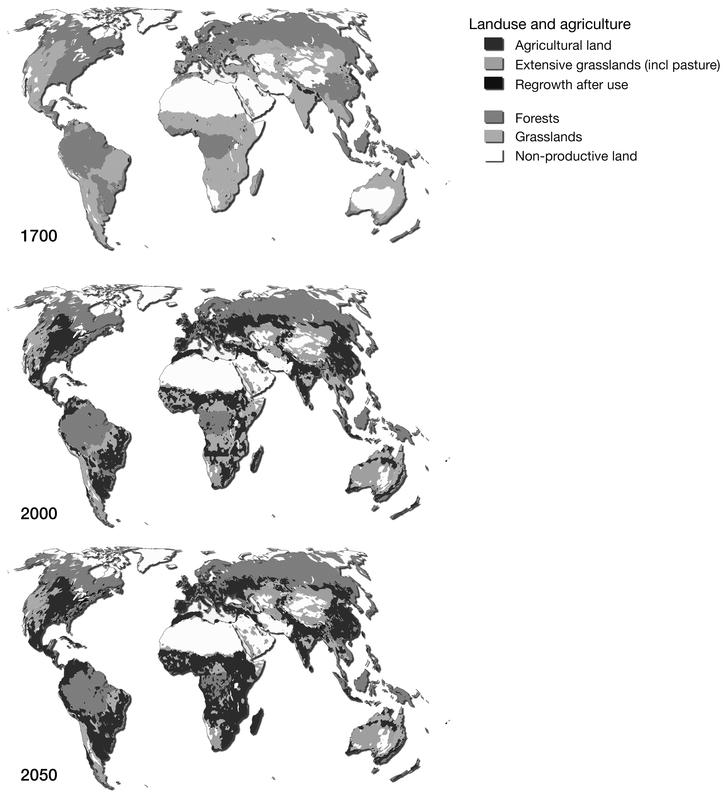
Figure 10.1a Land use and agriculture and biodiversity loss.
These rapid changes are problematic for the health of humans, other species and the environment. Industrialisation of the food chain has contributed to a global incidence of overweight and obesity of approximately 1.5 billion, despite some 1–2 billion people suffering from inadequate calorie or macro/micronutrient intake (Bhutta and Salam 2012; Nordin et al. 2013). Concurrently, the anthropogenically dominated landscape is profoundly impacting non-human species. Habitat loss and fragmentation is a primary threat to many endangered wild species, with agricultural expansion a major contributor (Baillie et al. 2004; Can et al. 2014). Between 1970 and 2014, animal species populations are estimated to have declined by 60 percent, with freshwater species declining by 83% according to the living planet index (WWF/ZSL 2018). The speed of decline indicates that a sixth mass-extinction is underway (Ceballos et al. 2005). Other human-influenced biotic and abiotic factors are also fundamentally changing the planetary geophysical and chemical systems. For example, global agriculture and human food production is estimated to contribute 25 per cent of all greenhouse gases, which are driving rapid warming of the planet’s climate (Edenhofer et al. 2014). Climate change is predicted to have far-ranging impacts across the biosphere, with a recent meta-analysis estimating that around one in six (16 per cent) species could become extinct under current policies (Urban 2015). Conservation efforts have had limited success, but they have not reversed the current rate of biodiversity loss (Hoffman et al. 2010) (Figure 10.1b).
Competition between humans and non-human animals over resources such as food and space damages both populations. Human–wildlife conflict arising through depredation of livestock by large predators (Boitani et al. 2015) or agricultural crops by herbivores such as elephants (Mamo et al. 2015) negatively impacts on farmer livelihoods and often results in retaliatory attacks on wildlife. Further, encroachment of humans and livestock on wildlife habitat has created new opportunities for emergence and exchange of infectious agents. Scientists have identified an increase in new and emerging infectious diseases in humans, a majority of which had their origins in non-domesticated animals (Jones et al. 2008; Morse et al. 2012). Many such pathogens are now independently maintained in humans (e.g. human immunodeficiency virus, HIV) and their domestic animals (e.g. highly pathogenic avian influenza, HPAI). Changing human food production systems as a key driver for disease emergence is relatively well understood (Jones et al. 2013; Keesing et al. 2010; McFarlane, Sleigh and McMichael 2013), but rarely have the resource-related needs of non-human animals been discussed in this context.
In this chapter, we investigate the biological dimensions of conflict between humans and non-human animals. Human–wildlife conflict is any interaction between humans and wildlife where the needs and behaviour of wildlife negatively impact the goals, needs and behaviours of humans, and vice versa (Cline, Sexton and Stewart 2007; Madden 2004). Infectious diseases are an example of a negative impact as a result of interaction between species. Competition over resources – and food in particular – is a key driver of human–wildlife conflict and disease emergence. Using four case studies ranging from sociobehavioural and habitat-related issues to the nutritional preferences of animals, we show how diverse synthesis can improve understanding of the complex nature of this topic and influence the development of strategies that facilitate the cohabitation of human and non-human animals.
The carnivores
Our first case study explores the African wild dog (Lycaon pictus; AWD) in carnivore–human conflict, noting its relevance to other species. When carnivores come into conflict with humans – worldwide – they are more vulnerable to extinction (Ripple et al. 2014). Strict carnivores such as large cats (Felidae) and wolves (Canis lupus) cause livestock depredation which is a major cause of human–wildlife conflict (Chapron et al. 2014; Inskip and Zimmermann 2009; Ripple et al. 2014; Treves and Karanth 2003). The impact of livestock depredation and fear of carnivores can lead to negative public perception and persecution (Zedrosser et al. 2011). In Nepal, endangered snow leopards (Panthera uncia) attacked livestock raised by resident agro-pastoralists, resulting in an estimated loss of more than US$44,000 in less than two years, culminating in retaliatory killings of snow leopards and other predators (Aryal et al. 2014). Situations such as these are a global problem involving several carnivore species, ranging from wolverine (Gulo gulo) in Scandinavia (Boitani et al. 2015) to dingoes (C. l. dingo) and domestic crosses in Australia (Fleming et al. 2001).
Case study 1: African wild dogs (AWD)
AWDs belong to a unique genus (i.e. distinct from Canis) within the dog family (Canidae) and are proficient predators, hunting in packs, never scavenging. They were once a successful species, ranging across the savannahs of Africa where they thrived on abundant antelope prey for over a million years (Savage 1978). With the evolution and expansion of humans and associated livestock and the decline of wild herbivore populations, this wide-ranging species is now extinct in all but a few locations; today it is classified as endangered on the International Union for Conservation of Nature (IUCN) 2015 Red List (Woodroffe and Sillero-Zubiri 2012). Research on causes of mortality in free-ranging populations of AWD across Africa showed that 73 per cent of adults and 16 per cent of pups died from human-caused road/train accidents, shooting, poisoning and snaring (Woodroffe, McNutt and Mills 2004). While deaths occur inside and outside of protected areas the majority of the conflict is in the community or privately owned lands. The wild dog is particularly susceptible to this, as their home range usually exceeds that of the available protected areas. A further 5 per cent die from diseases, such as rabies and canine distemper contracted from the domestic dog reservoir (Woodroffe, McNutt and Mills 2004). Insufficient protected land for this species and subsequently competition over resources is at the root of the conservation challenge. The recent history of the WDA provides an excellent illustration of depredation and human food–wildlife conflict.
In wildlife protected, private or community wildlife areas AWD are mostly welcomed, and considered important for eco tourism (Lindsey et al. 2005). In game ranches where hunting, game consumption and livestock farming occur, a perception exists that the AWD compete with other species for trophy animals or food (Lindsey et al. 2005). Research shows that AWDs tend to select weak and poor trophy animals suggesting that, far from being a competitor, they provide an ecosystem service by ensuring selection pressure for better quality animals (Pole et al. 2004). Studies also show that AWDs are less relevant in this conflict than the lion, hyena and leopard (Ogada et al. 2003; Woodroffe et al. 2005), and that livestock are more likely to die due to disease or theft than depredation (Frank 1998; Rasmussen 1999). Nevertheless, there are documented examples of livestock losses due to AWD affecting both extensive community systems and more intensive livestock systems and ranches (Davies and du Toit 2004; Kock et al. 1999; Rasmussen 1999; Woodroffe et al. 2005).
A survey of attitudes among Southern African livestock and game farm owners (mostly ranchers from ethnically European, English and Afrikaans speaking communities) provides insight into the problem (Lindsey et al. 2005). Notably, depredation by AWD appears to be worse with small and fenced properties, or where offtake for commercial purposes was high, requiring artificially high densities of livestock. In open systems, such as conservancies (i.e. multiple properties cooperating and removing fencing) with less restrictions on animal movement, the presence of AWDs was better tolerated. In more traditional African communities, such as pastoral systems in Eastern Africa, nomadic livestock keepers tolerate the AWD even though the level of cattle husbandry is high. Protection is via the use of fortified enclosures (‘bomas’), guarded by humans and dogs, and more recently with the use of ‘lion lights’ for night protection (Ogada et al. 2003; Pimm 2012). But there are communities in Eastern Africa – mainly agro-pastoralists with settled livestock – that are more likely to persecute AWDs when they pass through their land. They fear the animal and suffer from raids in the absence of protective measures (Kock et al. 1999).
The usual behaviour of AWD in areas of abundant herbivorous wildlife is to select natural prey species and avoid contact with humans and livestock. The loss of natural prey in human settled environments is associated with increased depredation – previously reported in southern Africa (Woodroffe et al. 2005). Sometimes AWDs occupy community lands despite adjacent protected areas having abundant prey, perhaps reflecting competition pressures with other larger carnivores. Lions seek out and kill AWD (Kock et al. 1999) and, in protected areas like the Serengeti National Park (SNP), lions survive in high densities where they are effectively over-protected by the absence of humans. In contrast, AWD was extirpated from the SNP in the late 1980s, and only small populations were rarely observed to survive on the periphery.1 Further, learned behaviour around livestock (i.e. modified based on experience) occurs given the inability of some domestic animals to respond appropriately to predator attacks (Rasmussen unpublished. Data in: Woodroffe, McNutt and Mills 2004). One account describes a small pack of young AWDs – a breakaway group from larger packs present on the lowlands – that learned to attack flocks of merino sheep on the slopes of Mount Kenya (Kock personal observation 1996; Kock et al. 1999). The sheep tended to form a compact unit rather than flee when under attack, resulting in a frenzy of killing or maiming, with no attempt to feed on or remove the carcasses. This behaviour was rarely if ever reported in the pastoral lands, where sheep and goats are more feral. As with other carnivores, learned behaviours around predation of livestock (or humans) are usually fatal to the pack or individual and therefore self-limiting. Evidence suggests that learning is also affected by the individual needs of the animal, such as lack of prey or from degradation of the habitat making hunting difficult. The intensification of agricultural systems, crop agriculture alongside rangelands, artificially high prey densities in game ranches, fencing systems and human settlements all influence AWDs ranging and hunting behaviours. These landscape changes degrade the ecosystem and create sinks – areas where losses produce a vacuum for expansion of adjacent populations – sometimes leading to overall decline in a species population (Delibes, Gaona and Ferreras 2001).
Sharing the land
Because humans inhabit the same area as domesticated and non-domesticated animals, strategies are required to better manage wildlife that forage in anthropogenic environments. There have been some successes. In Europe, large carnivore populations are on the increase despite close proximity to humans, due to management practices and positive public perception (Chapron et al. 2014). However, in addition to increased human–wildlife conflict, close proximity brings risk of disease to all involved. Coyotes (Canis latrans) which forage in urban areas around North America, for example, tend to have higher incidences of sarcoptic (Sarcoptes scabiei) mange (Murray et al. 2015).
Case study 2: Bat to basics
Sharing habitat with wildlife is particularly germane to highly mobile, aerial species such as birds and flying mammals. As a keystone species, fruit bats – such as those belonging to the genus Pteropus (‘flying foxes’) – provide essential ecosystem services including seed dispersal and pollination (McConkey et al. 2006). Flying foxes respond to food supply by migrating throughout the landscape and occupying specific sites based on proximity to preferable food sources (Schmelitschek, French and Parry-Jones 2009). They roost in camps during the day and feed at night. Fruit bats are considered ‘sequential specialists’, meaning they feed on items in a hierarchy of preference until they are depleted or seasonally unavailable (Parry-Jones and Augee 2001). The grey-headed flying-fox (P. poliocephalus), on the east coast of Australia, displays a preference for nectar and pollen from eucalypts, melaleucas and banksias (Department of Environment, Climate Change and Water [DECCW] 2009; Parry-Jones and Augee 2001). When these are unavailable, dietary intake of forest fruits increases, with native figs (Ficus spp.) becoming a major component of the seasonal diet (Parry-Jones and Augee 2001; Schmelitschek, French and Parry-Jones 2009).
Along with numerous other Pteropus species, P. poliocephalus is listed as vulnerable on the IUCN 2015 Red List owing to continuing population declines (Lunney, Richards and Dickman 2015). In contrast to the AWD, where habitat fragmentation and conflict with humans is the primary threat (Woodroffe and Sillero-Zubiri 2012), loss of foraging and roosting habitat is the primary threat to P. poliocephalus (Duncan et al. 1999). One study which examined the full geographic extent of P. poliocephalus found 50 per cent loss of native vegetation (Eby and Law 2008). Although stone fruit are relatively low in the feeding hierarchy (Parry-Jones and Augee 2001), flying foxes feed on commercial fruit crops when native food sources are scarce which brings them into conflict with orchardists (DECCW 2009).
Loss of native habitat and urban expansion (Markus and Hall 2004), as well as anthropogenic effects on local climate (Parris and Hazell 2005), may be influencing this historically temperate/tropical forest-dwelling species to utilise southern, urban environments. The number and size of urban flying-fox camps has increased (Tait et al. 2014), with some urban camps occupied year round (Parry-Jones and Augee 2001; van der Ree et al. 2006). Urbanisation may be a behavioural response to the advantages offered by this landscape (Tait et al. 2014); since European settlement there has been a dramatic increase in availability of food in urban centres (Williams et al. 2006), with figs in particular providing a year round source at some sites (Parry-Jones and Augee 2001). Street trees in suburban areas appear to have had nutritional benefit for flying foxes (McDonald-Madden et al. 2005), but it comes at a cost of increased interaction with humans and domesticated animals.
Flying foxes have been implicated as the host to several newly discovered viruses of public health concern (Table 10.1). While bats may be unique for their ability to tolerate some of the most deadly viral zoonoses known (Wang, Walker and Poon 2011), the sudden spillover to humans and other intermediate hosts – and thus discovery of these viruses – appears to be driven by factors other than the presence of these viruses in fruit bats. For example, efforts to understand why Hendra virus (HeV) recently emerged in Australia have centred around the changing behaviour and feeding ecology of flying foxes. HeV is spread from flying foxes to horses, and from horses to humans. Since horse and human population density are related (McFarlane, Becker and Field 2011), urban aggregation of flying foxes increases opportunities for contact with horses (Plowright et al. 2011). Indeed, spatial analysis reveals clustering of equine HeV cases at around 40 km – consistent with nocturnal foraging range of flying foxes (Smith et al. 2014). Risk of HeV transmission to horses is also known to increase during periods of flying-fox reproduction and nutritional stress. In one study, bats sampled during a blossom and nectar shortage had a 14–42 times higher odds of seropositivity, compared to bats studied in other seasons (Plowright et al. 2008). The high seroprevalence in this context may be due to increased viral susceptibility or an adaptive behavioural response to nutritional stress, such as crowding around restricted food sources or sharing food with other bat species that are more competent at transmitting HeV. Further research indicates that decreased migratory behaviour also affects infection dynamics; with a lower probability of HeV reinfection of camps, the proportion of immunologically naive (i.e. never before exposed) offspring accumulates. When HeV is subsequently reintroduced, high-intensity outbreaks follow, resulting in spillover to horses and potentially humans (Plowright et al. 2011).
|
Virus |
Drivers |
|---|---|
|
Severe acute respiratory syndrome-coronavirus (SARS-CoV) |
Economic growth Desire for game meat Live wild animal trading in wet markets International travel |
|
Ebola virus |
Desire for game meat Live wild animal trading Burial practices |
|
Marburg virus |
Infected monkeys used for research Mining Tourism |
|
Hendra virus |
Population growth/urbanisation/human encroachment/synanthrophy Climate change Starvation Reproductive stress |
|
Nipah virus (Bangladesh) |
Date palm juice (food source) Cultural tradition |
|
Nipah virus (Malaysia) |
Agricultural intensification (dual land use) Encroachment into forested areas Movement of pigs to grower piggeries within Malaysia Food processing in Singapore Trade Habitat destruction Stress |
|
Lyssaviruses, e.g. rabies, Australian bat lyssavirus (ABLV) |
Urbanisation Deforestation Synanthrophy |
Table 10.1. Drivers for selected emerging bat zoonotic viruses. Adapted from Smith and Wang 2013.
Similar links between bat foraging behaviour, land use changes, and disease spillover have been established for Nipah virus (NiV). In this instance, emergence in Malaysia was facilitated by the practice of growing fruit trees (particularly mango) alongside pigsties (an example of ‘dual-use agriculture’), thus creating opportunities for contact between the two species. Further, repeated introductions of NiV into the index farm – a high-turnover, commercial pig farm (i.e. with continuous supply of naive hosts) – allowed the virus to persist, leading to increased transmission among pigs, and from pigs to humans (Pulliam et al. 2012). Thus, anthropogenic events and actions that alter food availability to flying foxes – such as habitat loss, urbanisation and specific agricultural practices – are key reasons why emerging diseases like HeV and NiV have suddenly appeared in the human population (Plowright et al. 2008).
Case study 3: Water wars: wild birds, poultry and people
Pigs and poultry are particularly adaptable to industrial farming systems, since they can tolerate high-grain diets and more efficiently convert this feed into edible products such as meat and eggs compared to ruminants (Food and Agriculture Organization of the United Nations [FAO] 2009; O’Mara 2012). These markets have undergone rapid expansion in recent decades, consistent with the shift away from pasture-based farming systems and rising demand for affordable meat (FAO 2009; Steinfeld et al. 2010) (Figure 10.2). Although industrial farming systems were developed in the West, much of the growth in livestock production is now occurring in low- and middle-income countries (the so-called livestock Revolution [Delgado et al. 1991]) many of which ship products to the high-income countries.
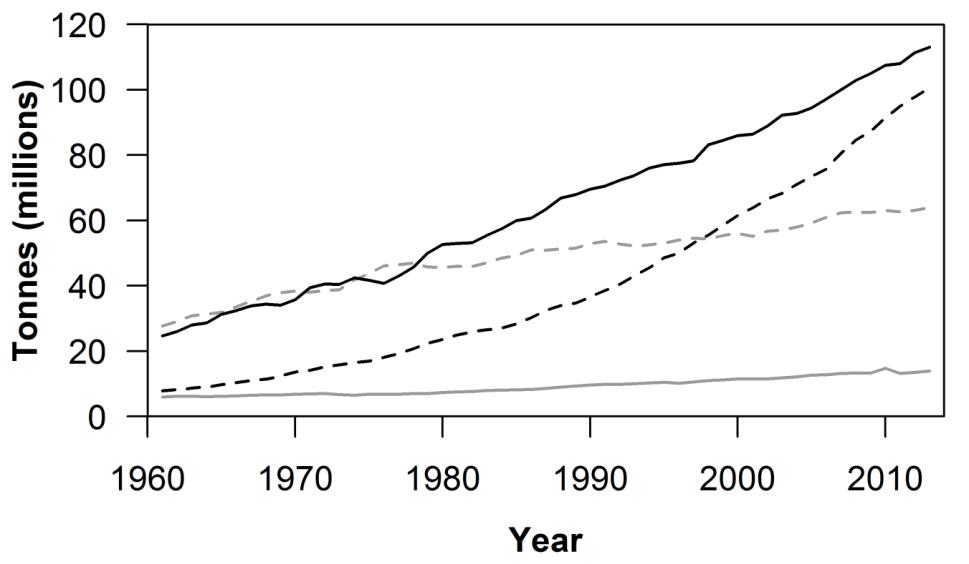
Figure 10.2 Global production of meat, by type, 1961–2013. The plot depicts total global production of meat from cattle (grey-stippled line), sheep and goats (grey-solid line), pigs (black-solid line) and poultry, including chickens and ducks (black-stippled line). Data from FAOSTAT 2014.
An efficient commercial poultry industry requires feed and water to be delivered to birds with precision throughout the year (FAO 2009). This contrasts with migratory wild birds which move vast distances to access climatic and feed resources to support their life cycles (Chu 2007). Migratory bird flyways link areas where birds can find fresh water and feed, including lake, pond and riverine habitats. The dramatic increase in commercial poultry production has resulted in more fresh water resources being used to supply commercial poultry units (FAO 2009), contributing to the 87 per cent of fresh water reserves now consumed globally for agriculture (Postel, Daily and Ehrlich 1996). Commercial poultry farms in Australia have used treated water for their birds for years (Department of Agriculture, Fisheries and Forestry 2009) but this treatment is not applied uniformly throughout the world, notably where human water supplies are untreated. Free-range production of poultry (i.e. ducks in Asia and layer chickens in many parts of the world) has provided new opportunities for sedentary and migratory birds to source their feed and water from the same supplies (FAO 2008).
This resource conflict between commercial birds and wild birds has facilitated the emergence of novel strains of the influenza virus. Low pathogenic avian influenza (LPAI) virus occurs naturally in populations of wild and domesticated birds without signs of clinical disease (Alexander 2007; Capua and Alexander 2009). Outbreaks of HPAI in commercial chickens have occurred in Australia (Bulach et al. 2010) and elsewhere (Alexander 2007) when untreated fresh water became contaminated with LPAI from wild bird faeces. The genetic homogeneity and high-density production of the commercial chicken industry favours genetic drift or reassortment of low pathogenic strains of avian influenza into high pathogenic strains (Alders et al. 2014). The HPAI subtype H5N1 – which causes fatal infections in humans – emerged in conjunction with the massive increases in both human and commercial chicken and duck populations in Asia. The commercial duck population increased five-fold during the two decades prior to the H5N1 outbreak in China (Li et al. 2015). These commercial operations (poultry production and rice cultivation) utilised natural and semi-natural waterbodies that were occupied permanently or seasonally by wild birds. The practice of applying untreated animal waste on land (as fertiliser) and in ponds (as fish feed in aquaculture) compounded the problem because undigested grains are an attractant to wild birds (Graham et al. 2008).
The nutritional dimension
Many animals, including bats and wild birds, migrate or move between habitats to obtain food. While the focus on foods is informative, recent research shows that understanding and predicting the behaviour of animals (including humans) based on the combinations of nutrients in foods and diets is valuable (Simpson and Raubenheimer 2012). The relative balance of macronutrients (fats [F], carbohydrates [C], and proteins [P]) in foods has been shown to exert a dominant influence in this regard. The macronutrient preferences of strict carnivores are relatively high in protein, followed by lipids, while carbohydrate preferences are relatively low (Kohl, Coogan and Raubenheimer 2015). One study showed the diet of feral cats (Felis catus) was composed of 52P:46F:2C (per cent of energy) from wild prey (Plantinga, Bosch and Hendriks 2011) while wild wolves had a diet of 54P:45F:1C (Bosch, Hagen-Plantinga and Hendriks 2015). The macronutrient ratios selected by domestic cats (F. catus) in controlled experimental studies involving manufactured feeds (Hewson-Hughes et al. 2013) were similar to the ratios selected by feral cats in the wild, demonstrating that these ratios are specifically targeted rather than due to constraints on available foods in the wild. Many species of omnivores and herbivores likewise regulate their macronutrient intake, typically to lower ratios of protein than carnivores (Simpson and Raubenheimer 2012). Micronutrients (e.g. essential minerals) also play a role in animal foraging and migratory behaviour, as observed in the giant panda (Ailuropoda melanoleuca; Nie et al. 2015). Such nutrient-specific foraging may be useful in predicting and understanding why animals target certain foods.
Case study 4: Anthropogenic food-related human–bear conflict
Both black (Ursus americanus) and grizzly (U. arctos) bears come into conflict with humans in North America, often through livestock depredation (Gunther et al. 2004; Witmer and Whittaker 2001). In contrast to strict carnivores, both black and grizzly bears are omnivorous carnivores and consume a wide range of anthropogenic foods and garbage (Baruch-Mordo et al. 2008; Can et al. 2014; Follmann and Hechtel 1990; Gunther et al. 2004). Yellowstone National Park (YNP), has a relatively long anthropogenic food-related history with bears (Craighead, Sumner and Mitchell 1995; National Park Service [NPS] 2008). From the turn of the 20th century to the 1940s, visitors to YNP were entertained by ‘bear shows’ – spectators would gather around garbage dumps to watch bears forage on human discards (Craighead, Sumner and Mitchell 1995; NPS 2008). By the 1970s, the population density of bears in the park was high, with bears attaining relatively large body sizes. When a bear management strategy that included closing garbage dumps in and around the park was introduced in the 1970s, the grizzly bear population decreased substantially and incidences of human–bear conflict greatly increased, resulting in over 140 human-caused grizzly bear mortalities (Craighead et al. 1988; Craighead, Sumner and Mitchell 1995; NPS 2008). Access to large amounts of garbage in other regions has also resulted in high-density populations of relatively large bears including with shorter foraging and denning periods (Baldwin and Bender 2010; Beckmann and Berger 2003). The bear population in YNP has again increased (NPS 2008), and while grizzly bears are currently a threatened species in the USA (FWS 1975) the Yellowstone population was recently, and controversially, considered for delisting (Doak and Cutler 2014; Morello 2014); a decision to delist had not been made at the time of writing. The controversy centres in large part on a key food of grizzly bears – the seeds of white bark pine (WBP) (Pinus albicaulis). White bark pine is in decline due to climate-induced changes in the occurrence of a pest which kills the tree, leading to concerns that its loss will negatively impact the bear population.
In North America, human–bear conflict incidents increase from spring through the early and late autumn “hyperphagic” season (Gunther et al. 2004) when bears attempt to eat enough to gain sufficient fat and lean mass to support hibernation, and, for females, reproduction (Lopez-Alfaro et al. 2013). In the Greater Yellowstone Ecosystem (GYE) incidents of bears obtaining anthropogenic foods, raiding gardens/orchards for fruits and vegetables, and obtaining honey from domestic beehives and apiaries peak during late hyperphagia (September to den entrance), while livestock depredation peaks in early hyperphagia (mid-July to end August) (Gunther et al. 2004). Human–bear conflict also increases when natural foods are scarce, especially during the hyperphagic season (Gunther et al. 2004; Mattson, Blanchard and Knight 1992; Peine 2001). When the autumn availability of high-fat WBP seeds and army cutworm moths (Euxoa auxiliaris) in the Yellowstone ecosystem was poor, conflicts due to grizzly bears obtaining anthropogenic foods increased significantly (Gunther et al. 2004). Likewise, black bear–human conflicts in Tennessee rose dramatically in autumn following a late frost and summer drought which resulted in a autumn mast-crop failure (Peine 2001). These food-related conflicts often end tragically for bears. Incidences of human-caused grizzly bear mortality in the GYE increased when WBP seed production was low (Mattson, Blanchard and Knight 1992). Anthropogenic food-conditioned bears are more likely to damage property in search of food, and are now a problem for human communities close to wild bear populations (Peine 2001).
Bears are especially susceptible to anthropogenic food attractants which often leads to conflict with humans because being omnivorous, they have a preference for a higher dietary proportion of non-protein macronutrients relative to protein 17P:83non-P, (per cent of metabolisable energy) (Erlenbach et al. 2014). This means they feed on a wider range of carbohydrate- and fat-rich anthropogenic foods that are not attractive to strict carnivores (Coogan and Raubenheimer 2016) (Figure 10.3). Diets balanced in this way maximise mass gain in grizzly bears, which is an indicator of fitness. For example, bears need to gain enough fat and lean mass to endure long periods of hibernation without eating, and larger body size offers an advantage in dominance interactions for resources (e.g. mates and food). In the wild, bears forage on a range of naturally occurring foods which may allow them to mix their diet in optimal proportions (Coogan et al. 2014). Sources of carbohydrates and lipids are often limiting to wild bears relative to protein, and natural foods rich in non-protein macronutrients are generally only abundant during the late summer and autumn periods, such as fruit and hard mast (e.g. seeds and nuts of trees and shrubs). In the absence of suitable natural foods (such as during a berry crop failure in the autumn) anthropogenic food sources can offer bears a rich source of carbohydrates and lipids which may optimise their diet prior to hibernation and likely exacerbates bear–human conflict during this time (Coogan and Raubenheimer 2016). The nutritional preferences of animals, and the ability for anthropogenically altered environments to provide those preferences, requires serious consideration as the human environment continues to encroach on wild habitats.
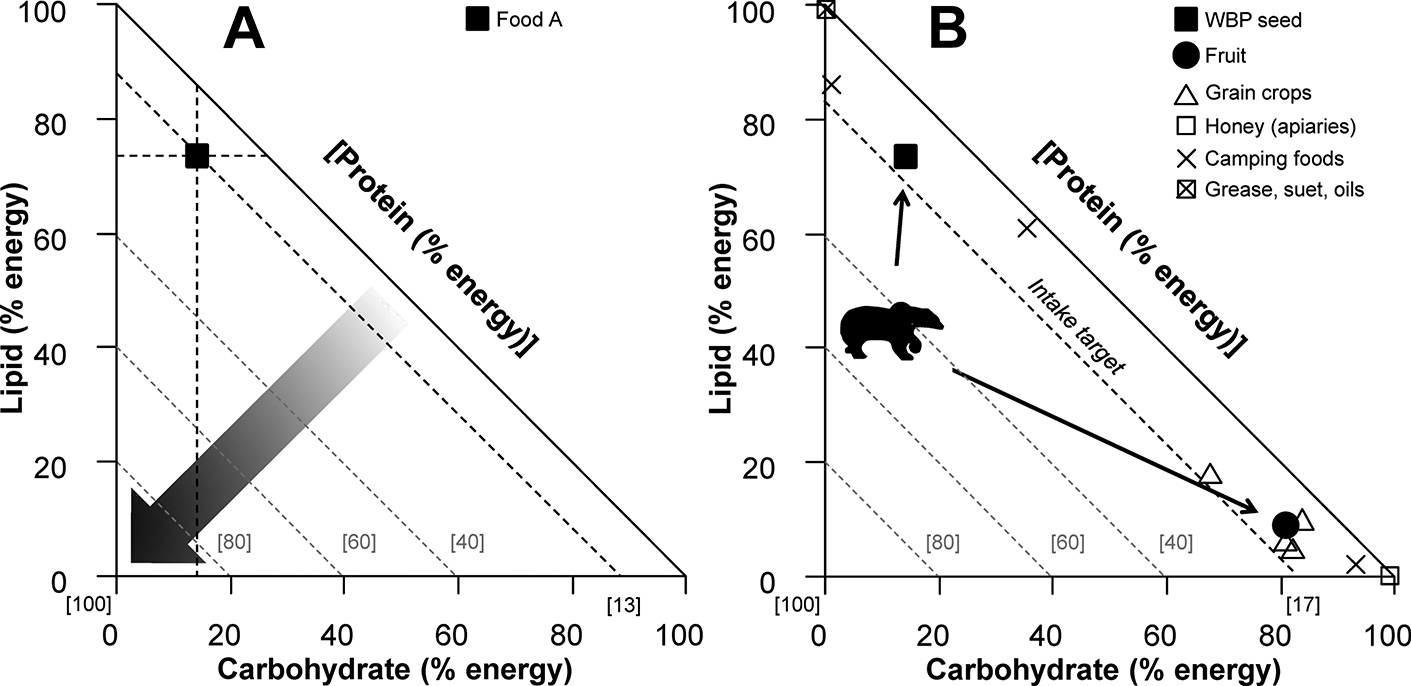
Figure 10.3 Panel A: right-angled mixture triangle illustrating the macronutrient (carbohydrate, lipid, and protein) composition of a hypothetical food (Food A) as a percentage of energy derived from the sum of these components. Food A contains approximately 73 per cent lipid and 14 per cent carbohydrate. The remaining 13 per cent of energy from protein is read on the implicit axis (negatively sloped black dashed line). Any mixture of macronutrients that falls along this line contains 13 per cent protein energy with non-protein energy varying accordingly. The value of the implicit axis is inversely related to the plot origin, as illustrated by the arrow with a gradient showing values of protein from low (white) to high (black). Dashed-grey lines at 40, 60 and 80 per cent protein energy have been added for reference. Panel B: we demonstrate how the macronutrient composition of foods can be used to understand patterns of human–wildlife conflict using data for the grizzly bear (Ursus arctos). The ratio of protein to non-protein energy (intake target) that was self-selected by grizzly bears in a captive trial is plotted (17 per cent protein:83 per cent non-protein energy). Grizzly bears generally consume a diet high in protein relative to their preferences (as demonstrated by the bear icon) until late summer and fall when highly sought after high-fat or high-carbohydrate foods such as white bark pine (WBP) seed and fruit are available. By consuming these foods (black arrows) bears are able to reach their intake target thereby optimising their mass gain before hibernation. When such foods are scarce or unavailable, grizzly bears may consume anthropogenic foods high in non-protein energy which may lead to increased incidences of conflict with humans. To illustrate, the nutrient composition of a selection of anthropogenic foods which grizzly bears have been documented to feed upon is shown. Figure adapted from Coogan and Raubenheimer 2016.
The future
These examples show how unintended consequences arise when the human appetite for resources impinges on the requirements of other species. With human population projected to grow to 9.7 billion by 2050, modelling has suggested that a 70 per cent increase in food production will be required to meet human food requirements (FAO 2009). This is seemingly inevitable, unless food waste can be reduced significantly, which currently amounts to about 40 per cent of production. This would also more or less cancel out the over-consumption, and under-consumption, of food in different parts of the world thereby improving the health of all. Importantly, reducing food waste will reduce the need to expand agriculture, and thus contribute to reducing or reversing the decline in biodiversity. The changing global climate, increasing economic growth, and resulting ecological impact are compounding challenges. A business-as-usual approach that fails to consider the resource needs of other species will extend human–wildlife conflict, accelerate species extinction and the spillover of infectious diseases from non-human animals to humans. These examples show that conflicting appetites are best managed using an integrated ecological approach, one that moves beyond the simplistic dichotomy of ‘anthropogenic vs natural’ and considers all species, including humans, domesticated and un-domesticated animals, as participants in an increasingly globalised ecology.
One of the most challenging and instructive scenarios involves predators. In terrestrial environments, large predators are not typically being threatened because they are harvested for food, but rather because of their large habitat requirements, depleted food base, and the threat they pose to humans and associated domesticated species (i.e. persecution). Because predators play an important role in ecosystems (Schmitz and Suttle 2001) it is imperative that conflict situations are better managed. In the case of the AWD, where protected areas are insufficient, the most effective strategies have included more open landscape management (conservancies), with multiple land use and ownership, including wildlife economy and livestock-based agriculture (Lindsey et al. 2005). By conserving a broad range of resources from pastures to bushland or forests, food sources are available for the prey species and habitat for the carnivores. This strategy combined with community conservation actions will have benefits such as losses from depredation compensated by alternative income sources. An integrated approach that looks after the interests of animals, humans and the environment provides the best hope of a future for the AWD and other carnivores in Africa. In this way, conservationists can improve the population status of wildlife species, as well as the livelihoods and diets of malnourished communities that share the landscape with African wildlife.
The bat and bird examples illustrate how the high mobility of some species ensures contact with humans and domesticated species. In both cases, the potential conflict is not competition for food (although this is a dimension in the human interaction with fruit bats), but rather complex multi-species interactions in a shared environment, involving humans, domesticated and non-domesticated animal hosts and their microbes. For bats, transmission of infectious agents is facilitated by depletion of their natural habitat and/or establishment of attractive alternatives within urban and agricultural environments. For migratory birds, the mode of contact is principally indirect, involving the shared use of riparian and aquatic habitats by non-domesticated bird species and high-intensity poultry production for human consumption. Whereas rich biodiverse communities tend to have a sterilising effect (so-called dilution effect), the placement of high-density, homogenous (farmed) animal communities adjacent to complex systems breaches biological norms and creates opportunities for species jumps and microbial evolution in new hosts (Civitello et al. 2015; Keesing et al. 2010). In the case of HeV, NiV, H5N1 influenza, and other pathogens, the results can be devastating to domesticated animals and pose a serious threat to humans. To be successful in the long term, intensive livestock production will require improved biosecurity and biocontainment practices as well as waste management strategies. These are essential for preventing zoonotic transfer of pathogens from animals in high-density settings (Graham et al. 2008). The role and significance of wildlife–livestock interface in disease ecology has been neglected; more research and surveillance on specific interfaces is warranted to mitigate the risk of disease emergence in humans (Alders and Kock 2017; Kock 2018; Wiethoelter et al. 2015). This is a challenge for low- and middle-income countries where veterinary and wildlife infrastructure is often weak and unable to predict or contain emerging disease events due to inadequate capacity and funding. These are the very same areas where pig and poultry production is on the increase, especially in tropical and subtropical environments, where microbial diversity is rich and potential for species jumping is high.
A recurring theme is conflict over shared resources, particularly foods. The case of the grizzly bear shows they are not in competition with humans for food per se but for specific combinations of nutrients on which they rely. When particular nutrients are scarce because of human impacts or ecological stochasticity, we can predict that anthropogenic sources of those specific nutrients, rather than foods per se, will provide a flashpoint for bear–human conflict. This conflict can be reduced by minimising the availability of, or reducing access to, the targeted nutrients in bear habitat (e.g. fruit orchards). Omnivores like bears use a wide range of foods as interchangeable sources of nutrients. The same reasoning might apply to crop raiding by herbivores such as elephants (Koirala et al. 2016), and carnivores more generally (Kohl, Coogan and Raubenheimer 2015). Understanding specific needs of the species with which we interact is an essential starting point for finding solutions to the conflicts that arise over competition for resources.
The main drivers of wildlife conservation issues are likely to be locally case-specific, involving different environmental, cultural, economic and geopolitical circumstances and requiring multidisciplinary effort to resolve. What is clear is that the old conservation paradigm of protected areas is now totally insufficient to maintain biodiversity and society needs to revisit integrated systems, where humans share landscapes with wildlife. This effort is required if we are to coexist with non-human species in this human dominanted landscape. As urban consumers become increasingly remote from their food sources (Satterthwaite, McGranahan and Tacoli 2010), we have to advocate for food value chains and extractive industry practices that ensure access to nutritionally balanced, ecologically sustainable diets for all species.
Works cited
Alders, R., J.A. Awuni, B. Bagnol, P. Farrell, and N. de Haan (2014). Impact of avian influenza on village poultry production globally. EcoHealth 11: 63–72. doi: 10.1007/s10393-013-0867-x.
Alders, R., and R. Kock (2017). What's food and nutrition security got to do with wildlife conservation?. Australian Zoologist 39: 120-126. doi: 10.7882/AZ.2016.040
Alexander, D.J. (2007). Summary of avian influenza activity in Europe, Asia, Africa, and Australasia, 2002–2006. Avian Diseases 51: 161–6. doi: 10.1637/7602-041306r.1.
Aryal, A., D. Brunton, W.H. Ji, R.K. Barraclough, and D. Raubenheimer (2014). Human–carnivore conflict: ecological and economical sustainability of predation on livestock by snow leopard and other carnivores in the Himalaya. Sustainability Science 9: 321–9. doi: 10.1007/s11625-014-0246-8.
Baillie, J.E.M., et al. (2004). 2004 IUCN Red List of Threatened Species. A global species assessment. Cambridge, UK: The World Conservation Union. http://bit.ly/2BW4IJq.
Baldwin, R.A., and L.C. Bender (2010). Denning chronology of black bears in Eastern Rocky Mountain National Park, Colorado. Western North American Naturalist 70: 48–54. doi: 10.3398/064.070.0106.
Bar-On, Y.M., R. Phillips, and R. Milo (2018). The biomass distribution on Earth. Proceedings of the National Academy of Sciences USA 115: 6506-6511. doi: 10.1073/pnas.1711842115.
Baruch-Mordo, S., S.W. Breck, K.R. Wilson, and D.M. Theobald (2008). Spatiotemporal distribution of black bear–human conflicts in Colorado, USA. Journal of Wildlife Management 72: 1853–62. doi: 10.2193/2007-442.
Beckmann, J.P., and J. Berger (2003). Rapid ecological and behavioural changes in carnivores: the responses of black bears (Ursus americanus) to altered food. Journal of Zoology 261: 207–12. doi: 10.1017/S0952836903004126.
Bhutta, Z.A., and R.A. Salam (2012). Global nutrition epidemiology and trends. Annals of Nutrition & Metabolism 61: 19–27. doi: 10.1159/000345167.
Boitani, L., et al. (2015). Key actions for large carnivore populations in Europe. Rome: Institute of Applied Ecology. http://bit.ly/2E5tFUb.
Bosch, G., E.A. Hagen-Plantinga, and W.H. Hendriks (2015). Dietary nutrient profiles of wild wolves: insights for optimal dog nutrition? British Journal of Nutrition 113: S40-S54. doi: 10.1017/S0007114514002311.
Bulach, D., et al. (2010). Molecular analysis of H7 avian influenza viruses from Australia and New Zealand: genetic diversity and relationships from 1976 to 2007. Journal of Virology 84: 9957–66. doi: 10.1128/Jvi.00930-10.
Can, O.E., N. D’Cruze, D.L. Garshelis, J. Beecham, and D.W. Macdonald (2014). Resolving human–bear conflict: a global survey of countries, experts, and key factors. Conservation Letters 7: 501–13. doi: 10.1111/Conl.12117.
Capua, I., and D.J. Alexander (2009). Avian influenza infection in birds: a challenge and opportunity for the poultry veterinarian. Poultry Science 88: 842–6. doi: 10.3382/ps.2008-00289.
Ceballos, G., et al. (2015). Accelerated modern human-induced species losses: entering the sixth mass extinction. Science Advances 1: e1400253. doi: 10.1126/sciadv.1400253.
Chapron, G., et al. (2014). Recovery of large carnivores in Europe’s modern human-dominated landscapes. Science 346: 1517–9. doi: 10.1126/science.1257553.
Chu, M. (2007). Songbird journeys: four seasons in the lives of migratory birds. New York: Walker & Company.
Civitello, D.J., et al. (2015). Biodiversity inhibits parasites: broad evidence for the dilution effect. Proceedings of the National Academy of Sciences of the United States of America 112: 8667–71. doi: 10.1073/pnas.1506279112.
Cline, R., N. Sexton and S.C. Stewart (2007). A human-dimensions review of human–wildlife disturbance: a literature review of impacts, frameworks, and management solutions. US Geological Survey, Open-File Report 2007–1111. https://doi.org/10.3133/ofr20071111
Coogan, S.C.P., and D. Raubenheimer (2016). Might macronutrient requirements influence grizzly bear–human conflict? Insights from nutritional geometry. Ecosphere 7: e01204. doi: 10.1002/ecs2.1204.
Coogan, S.C.P., D. Raubenheimer, G.B. Stenhouse, and S.E. Nielsen (2014). Macronutrient optimization and seasonal diet mixing in a large omnivore, the grizzly bear: a geometric analysis. PLOS One 9: e97968. doi: 10.1371/journal.pone.0097968.
Craighead, J.J., K.R. Greer, R.R. Knight, and H.I. Pac (1988). Grizzly bear mortalities in the Yellowstone ecosystem 1959–1987. Bozeman: Montana Department of Fish Wildlife & Parks.
Craighead, J.J., J.S. Sumner, and J.A. Mitchell (1995). The grizzly bears of Yellowstone: their ecology in the Yellowstone ecosystem, 1959–1992. Washington, DC: Island Press.
Davies, H.T., and J.T. du Toit (2004). Anthropogenic factors affecting wild dog Lycaon pictus reintroductions: a case study in Zimbabwe. Oryx 38: 32–9. doi: 10.1017/S0030605304000067.
Delgado, C., M. Rosegrant, H. Steinfeld, and S. Ehui (1991). Livestock to 2020: the next food revolution. Food, Agriculture, and the Environment Discussion Paper 28. Washington, DC: International Food Policy Research Institute. http://bit.ly/2Sx80bC
Delibes, M., P. Gaona, and P. Ferreras (2001). Effects of an attractive sink leading into maladaptive habitat selection. American Naturalist 158: 277–85. doi: 10.1086/321319.
Department of Agriculture, Fisheries and Forestry (2009). National farm biosecurity manual – poultry production. Canberra: Australian Commonwealth Department of Agriculture, Fisheries & Forestry. https://bit.ly/2BLiuhA
Department of Environment, Climate Change and Water (2009). Draft national recovery plan for the grey-headed flying-fox Pteropus poliocephalus. Canberra: NSW Department of Environment Climate Change and Water. http://bit.ly/2zMuBK1.
Doak, D.F., and K. Cutler (2014). Re-evaluating evidence for past population trends and predicted dynamics of Yellowstone grizzly bears. Conservation Letters 7: 312–22. doi: 10.1111/Conl.12048.
Duncan, A., et al. (1999). The action plan for Australian bats. Canberra: Environment Australia. https://bit.ly/2SZdK1H
Eby, P., and B. Law (2008). Ranking the feeding habitats of grey-headed flying foxes for conservation management. http://bit.ly/2Ge6yJA.
Edenhofer, O., et al., eds. (2014). Climate Change 2014: mitigation of climate change. Working Group III contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. New York: Cambridge University Press.
Erlenbach, J.A., K.D. Rode, D. Raubenheimer, and C.T. Robbins (2014). Macronutrient optimization and energy maximization determine diets of brown bears. Journal of Mammalogy 95: 160–8. doi: 10.1644/13-Mamm-a-161.
Fleming, P., L. Corbet, R. Harden, and P. Thomson (2001). Managing the impacts of dingos and other wild dogs. Canberra: Bureau of Rural Sciences. http://bit.ly/2PnkPDd.
Foley, J.A., et al. (2005). Global consequences of land use. Science 309: 570–4. doi: 10.1126/science.1111772.
Follmann, E.H., and J.L. Hechtel (1990). Bears and pipeline construction in Alaska. Arctic 43: 103–9.
Food and Agriculture Organization Corporate Statistical Database (2014). FAOSTAT (online database). http://faostat3.fao.org/.
Food and Agriculture Organization of the United Nations (2008). Biosecurity for highly pathogenic avian influenza: issues and options. Rome: Food & Agriculture Organization of the United Nations. http://bit.ly/2BWrLnk.
Food and Agriculture Organization of the United Nations (2009). How to feed the world in 2050. Rome: Food & Agriculture Organization of the United Nations. http://bit.ly/2KZPVAp.
Food and Agriculture Organization of the United Nations (2009). The state of food and agriculture: livestock in the balance. Rome: Food & Agriculture Organization of the United Nations. http://bit.ly/2UjZQVL.
Frank, L.G. (1998). Living with lions: carnivore conservation and livestock in Laikipia District, Kenya. Bethesda, MD: Development Alternatives Inc. http://bit.ly/2Eixk1I.
Fish and Wildlife Service (1975). Endangered and threatened wildlife – grizzly bear. Federal Register 40: 31733–6.
Graham, J.P., et al. (2008). The animal–human interface and infectious disease in industrial food animal production: rethinking biosecurity and biocontainment. Public Health Reports 123: 282–99.
Gunther, K.A., et al. (2004). Grizzly bear–human conflicts in the Greater Yellowstone ecosystem, 1992–2000. Ursus 15: 10–22.
Hewson-Hughes, A.K., et al. (2013). Consistent proportional macronutrient intake selected by adult domestic cats (Felis catus) despite variations in macronutrient and moisture content of foods offered. Journal of Comparative Physiology B: Biochemical, Systems, & Environmental Physiology 183: 525–36. doi: 10.1007/s00360-012-0727-y.
Hoffmann, M., et al. (2010). The impact of conservation on the status of the world’s vertebrates. Science 330: 1503–9. doi: 10.1126/science.1194442.
Inskip, C., and A. Zimmermann (2009). Human–felid conflict: a review of patterns and priorities worldwide. Oryx 43: 18–34. doi: 10.1017/S003060530899030x.
Jones, B.A., et al. (2013). Zoonosis emergence linked to agricultural intensification and environmental change. Proceedings of the National Academy of Sciences of the United States of America 110: 8399–404. doi: 10.1073/pnas.1208059110.
Jones, K.E., et al. (2008). Global trends in emerging infectious diseases. Nature 451: 990-4. doi: 10.1038/Nature06536.
Kearney, J. (2010). Food consumption trends and drivers. Philosophical Transactions of the Royal Society B: Biological Sciences 365: 2793–807. doi: 10.1098/rstb.2010.0149.
Keesing, F., et al. (2010). Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature 468: 647–52. doi: 10.1038/Nature09575.
Kock, R.A. (2019) Is it time to reflect, not on the “what” but the “why” in emerging wildlife disease research?. Journal of Wildlife Diseases 55: 1-2. doi: 10.7589/2019-01-000.
Kock, R., J. Mwanzia, T. Fitzjohn, T. Manyibe, S. Kambe, and D. Lergoi (1999). African hunting dog translocation from Mount Kenya (Timau) to Tsavo West National Park Kenya 1996–1998. Nairobi: World Wildlife Fund.
Kohl, K.D., S.C.P. Coogan, and D. Raubenheimer (2015). Do wild carnivores forage for prey or nutrients. BioEssays 37: 701–9. doi: 10.1002/bies.201400171.
Koirala, R.K., W. Ji, A. Aryal, J. Rothman, and D. Raubenheimer (2016). Dispersal and ranging patterns of the Asian elephant (Elephas maximus) in relation to their interactions with humans in Nepal. Ethology, Ecology & Evolution 28: 221–31. doi: 10.1080/03949370.2015.1066872.
Li, X.L., et al. (2015). Highly pathogenic avian influenza H5N1 in mainland China. International Journal of Environmental Research & Public Health 12: 5026–45. doi: 10.3390/ijerph120505026.
Lindsey, P.A., R.R. Alexander, J.T. du Toit, and M.G.L. Mills (2005). The potential contribution of ecotourism to African wild dog Lycaon pictus conservation in South Africa. Biological Conservation 123: 339–48. doi: 10.1016/j.biocon.2004.12.002.
Lindsey, P.A., J.Y. du Toit and M.G.L. Mills (2005). Attitudes of ranchers towards African wild dogs Lycaon pictus: Conservation implications on private land. Biological Conservation 125: 113–121. doi: 10.1016/j.biocon.2005.03.015.
Lopez-Alfaro, C., C.T. Robbins, A. Zedrosser, and S.E. Nielsen (2013). Energetics of hibernation and reproductive trade-offs in brown bears. Ecological Modelling 270: 1–10. doi: 10.1016/j.ecolmode1.2013.09.002.
Lunney, D., G. Richards, and C. Dickman (2015). Pteropus poliocephalus. The IUCN Red List of Threatened Species, version 2015.1. www.iucnredlist.org/.
Madden, F. (2004). Creating coexistence between humans and wildlife: global perspectives on local efforts to address human–wildlife conflict. Human Dimensions of Wildlife 9: 247–57.
Mamo, D., H. Bouer, and Y. Tesfay (2014). Crop damage by African elephants assessment in Kaftasheraro National Park, Ethiopia. African Journal of Ecology 52: 138–43. doi: 10.1111/Aje.12094.
Markus, N., and L. Hall (2004). Foraging behaviour of the black flying-fox (Pteropus alecto) in the urban landscape of Brisbane, Queensland. Wildlife Research 31: 345–55. doi: 10.1071/Wr01117.
Mattson, D.J., B.M. Blanchard, and R.R. Knight (1992). Yellowstone grizzly bear mortality, human habituation, and whitebark-pine seed crops. Journal of Wildlife Management 56: 432–42. doi: 10.2307/3808855.
McConkey, K.R., and D.R. Drake (2006). Flying foxes cease to function as seed dispersers long before they become rare. Ecology 87: 271–6. doi: 10.1890/05-0386.
McDonald-Madden, E., E.S.G. Schreiber, D.M. Forsyth, D. Choquenot, and T.F. Clancy (2005). Factors affecting grey-headed flying-fox (Pteropus poliocephalus: Pteropodidae) foraging in the Melbourne metropolitan area, Australia. Austral Ecology 30: 600–8. doi: 10.1111/j.1442-9993.2005.01492.x.
McFarlane, R., N. Becker, and H. Field (2011). Investigation of the climatic and environmental context of Hendra virus spillover events 1994–2010. PLOS One 6(12): e28374. doi: 10.1371/journal.pone.0028374.
McFarlane, R.A., A.C. Sleigh, and A.J. McMichael (2013). Land-use change and emerging infectious disease on an island continent. International Journal of Environmental Research & Public Health 10: 2699–719. doi: 10.3390/ijerph10072699.
Morello, L. (2014). Yellowstone grizzlies face losing protected status. Nature 505: 465–6. doi: 10.1038/505465a.
Morse, S.S., et al. (2012). Prediction and prevention of the next pandemic zoonosis. Lancet 380: 1956–65. doi: 0.1016/S0140-6736(12)61684-5.
Murray, M., M.A. Edwards, B. Abercrombie, and C.C. St Clair (2015). Poor health is associated with use of anthropogenic resources in an urban carnivore. Proceedings of the Royal Society B: Biological Sciences 282: 20150009. doi: 10.1098/Rspb.2015.0009.
National Park Service (2008). Yellowstone grizzly bears. Yellowstone Science 16: 1–48.
Nie, Y.G., et al. (2015). Obligate herbivory in an ancestrally carnivorous lineage: the giant panda and bamboo from the perspective of nutritional geometry. Functional Ecology 29: 26–34. doi: 10.1111/1365-2435.12302.
Nordin, S.M., M. Boyle, T.M. Kemmer, and Academy of Nutrition and Dietetics (2013). Position of the Academy of Nutrition and Dietetics: nutrition security in developing nations: sustainable food, water, and health. Journal of the Academy of Nutrition & Dietetics 113: 581–95. doi: 10.1016/j.jand.2013.01.025.
Ogada, M.O., R. Woodroffe, N.O. Oguge, and L.G. Frank (2003). Limiting depredation by African carnivores: the role of livestock husbandry. Conservation Biology 17: 1521–30. doi: 10.1111/j.1523-1739.2003.00061.x.
O’Mara, F.P. (2012). The role of grasslands in food security and climate change. Annals of Botany 110: 1263–70. doi: 10.1093/Aob/Mcs209.
Parris, K.M., and D.L. Hazell (2005). Biotic effects of climate change in urban environments: the case of the grey-headed flying-fox (Pteropus poliocephalus) in Melbourne, Australia. Biological Conservation 124: 267–76. doi: 10.1016/j.biocon.2005.01.035.
Parry-Jones, K.A., and M.L. Augee (2001). Factors affecting the occupation of a colony site in Sydney, New South Wales, by the grey-headed flying-fox Pteropus poliocephalus (Pteropodidae). Austral Ecology 26: 47–55. doi: 10.1111/j.1442-9993.2001.01072.pp.x.
Peine, J.D. (2001). Nuisance bears in communities: strategies to reduce conflict. Human Dimensions of Wildlife 6: 223–37. doi: 10.1080/108712001753461301.
Pimm, S. (2012). Lion lights – a home grown solution to saving lions and livestock. http://bit.ly/2G6ekFq.
Plantinga, E.A., G. Bosch, and W.H. Hendriks (2011). Estimation of the dietary nutrient profile of free-roaming feral cats: possible implications for nutrition of domestic cats. British Journal of Nutrition 106: S35-S48. doi: 10.1017/S0007114511002285.
Plowright, R.K., et al. (2008). Reproduction and nutritional stress are risk factors for Hendra virus infection in little red flying foxes (Pteropus scapulatus). Proceedings of the Royal Society B: Biological Sciences 275: 861–9. doi: 10.1098/rspb.2007.1260.
Plowright, R.K., et al. (2011). Urban habituation, ecological connectivity and epidemic dampening: the emergence of Hendra virus from flying foxes (Pteropus spp.). Proceedings of the Royal Society B: Biological Sciences 278: 3703–12. doi: 10.1098/rspb.2011.0522.
Pole, A., I.J. Gordon, M.L. Gorman, and M. MacAskill (2004). Prey selection by African wild dogs (Lycaon pictus) in southern Zimbabwe. Journal of Zoology 262: 207–15. doi: 10.1017/S0952836903004576.
Postel, S.L., G.C. Daily, and P.R. Ehrlich (1996). Human appropriation of renewable fresh water. Science 271: 785–8. doi: 10.1126/science.271.5250.785.
Pulliam, J.R.C., et al. (2012). Agricultural intensification, priming for persistence and the emergence of Nipah virus: a lethal bat-borne zoonosis. Journal of the Royal Society Interface 9: 89–101. doi: 10.1098/rsif.2011.0223.
Rasmussen, G.S.A. (1999). Livestock predation by the painted hunting dog Lycaon pictus in a cattle ranching region of Zimbabwe: a case study. Biological Conservation 88: 133–9. doi: 10.1016/S0006-3207(98)00006-8.
Raubenheimer, D. (2011). Toward a quantitative nutritional ecology: the right-angled mixture triangle. Ecological Monographs 81: 407–27. doi: 10.1890/10-1707.1.
Ripple, W.J., et al. (2014). Status and ecological effects of the world’s largest carnivores. Science 343: 1241484. doi: 10.1126/Science.1241484.
Satterthwaite, D., G. McGranahan, and C. Tacoli (2010). Urbanization and its implications for food and farming. Philosophical Transactions of the Royal Society B: Biological Sciences 365: 2809–20. doi: 10.1098/rstb.2010.0136.
Savage, R.J.G. (1978). Carnivores. In Evolution of African mammals, V.J. Maglio and H.B.S. Cooke, eds., 249–67. Cambridge, MA: Harvard University Press.
Schmelitschek, E., K. French, and K. Parry-Jones (2009). Fruit availability and utilisation by grey-headed flying foxes (Pteropodidae: Pteropus poliocephalus) in a human-modified environment on the south coast of New South Wales, Australia. Wildlife Research 36: 592–600. doi: 10.1071/Wr08169.
Schmitz, O.J., and K.B. Suttle (2001). Effects of top predator species on direct and indirect interactions in a food web. Ecology 82: 2072–81. doi: 10.1890/0012-9658(2001)082[2072:Eotpso]2.0.Co;2.
Simpson, S.J., and D. Raubenheimer (2012). The nature of nutrition: a unifying framework from animal adaptation to obesity. Princeton, NJ: Princeton University Press.
Smith, C., C. Skelly, N. Kung, B. Roberts, and H. Field (2014). Flying-fox species density – a spatial risk factor for Hendra virus infection in horses in eastern Australia. PLOS One 9: e99965. doi: 10.1371/journal.pone.0099965.
Smith, I., and L.F. Wang (2013). Bats and their virome: an important source of emerging viruses capable of infecting humans. Current Opinion in Virology 3: 84–91. doi: 10.1016/j.coviro.2012.11.006.
Steinfeld, H., H.A. Mooney, F. Schneider, and L.E. Neville, eds. (2010). Livestock in a changing landscape: drivers, consequences, and responses. Washington, DC: Island Press.
Tait, J., H.L. Perotto-Baldivieso, A. McKeown, and D.A. Westcott (2014). Are flying-foxes coming to town? Urbanisation of the spectacled flying-fox (Pteropus conspicillatus) in Australia. PLOS One 9: e109810. doi: 10.1371/journal.pone.0109810.
Treves, A., and K.U. Karanth (2003). Human–carnivore conflict and perspectives on carnivore management worldwide. Conservation Biology 17: 1491–9. doi: 10.1111/j.1523-1739.2003.00059.x.
United Nations Population Fund (2011). The state of world population 2011. New York: United Nations Population Fund. http://bit.ly/2BUbnDU.
Urban, M.C. (2015). Accelerating extinction risk from climate change. Science 348: 571–3. doi: 10.1126/science.aaa4984.
van der Ree, R., M.J. McDonnell, I. Temby, J. Nelson, and E. Whittingham (2006). The establishment and dynamics of a recently established urban camp of flying foxes (Pteropus poliocephalus) outside their geographic range. Journal of Zoology 268: 177–85. doi: 10.1111/j.1469-7998.2005.00005.x.
Wang, L.F., P.J. Walker, and L.L. Poon (2011). Mass extinctions, biodiversity and mitochondrial function: are bats ‘special’ as reservoirs for emerging viruses? Current Opinion in Virology 1: 649–57. doi: 10.1016/j.coviro.2011.10.013.
Wiethoelter, A.K., D. Beltrán-Alcrudo, R. Kock, and S.M. Mor (2015). Global trends in infectious diseases at the wildlife–livestock interface. Proceedings of the National Academy of Sciences of the United States of America 112: 9662–7. doi: 10.1073/pnas.1422741112.
Williams, N.S.G., M.J. McDonnell, G.K. Phelan, L.D. Keim, and R. Van der Ree (2006). Range expansion due to urbanization: increased food resources attract grey-headed flying-foxes (Pteropus poliocephalus) to Melbourne. Austral Ecology 31: 190–8. doi: 10.1111/j.1442-9993.2006.01590.x.
Witmer, W.G., and D.G. Whittaker (2001). Dealing with nuisance and depredating black bears. Staff Publications 581. National Wildlife Research Center, United States Department of Agriculture. http://bit.ly/2rruwa1.
Woodroffe, R., P. Lindsey, S. Romanach, A. Stein, and S.M.K. ole Ranah (2005). Livestock predation by endangered African wild dogs (Lycaon pictus) in northern Kenya. Biological Conservation 124: 225–34. doi: 10.1016/j.biocon.2005.01.028.
Woodroffe, R., and C. Sillero-Zubiri (2012). Lycaon pictus. The IUCN Red List of Threatened Species, version 2015.1. www.iucnredlist.org/.
Woodroffe, R.B., J.W. McNutt, and M.G.L. Mills (2004). African wild dog. In Foxes, wolves, jackals and dogs: status survey and Conservation Action Plan, C. Sillero-Zubiri, M. Hoffmann, and D.W. Macdonald, eds., 174–83. Gland, CH: International Union for Conservation of Nature.
World Wildlife Fund (2018). Living planet report 2018. Gland, CH: World Wildlife Fund. http://bit.ly/2L0JkFQ.
Zedrosser, A., S.M.J. Steyaert, H. Gossow, and J.E. Swenson (2011). Brown bear conservation and the ghost of persecution past. Biological Conservation 144: 2163–70. doi: 10.1016/j.biocon.2011.05.005.
1 Recently AWD have been reintroduced into SNP and are now more commonly observed with at least two packs establishing. Ranging behaviour takes these animals in and out of the SNP and as far as the central Rift in Kenya. Losses continue when migrating these vast distances.