Chapter 2
Same words, different meanings: Learning to talk the scientific language of pharmacy
aFaculty of Pharmacy, bFaculty of Education and Social Work
Pharmacists are health care professionals whose expertise lies in the provision of medicines and information, with the aim of optimising medicine use in the overall care of patients. A strong foundation in both pharmaceutical and social sciences underpins the pharmacist’s role, and within the pharmacy curriculum at the University of Sydney relevant skills and attributes are embedded in this disciplinary knowledge. In relation to the pharmaceutical sciences, observations over a number of years (Sainsbury & Walker, 2004) suggested that many students experienced difficulties in applying communication, problem-solving and critical thinking skills to pharmacy issues, whereas those skills were clearly evident in other contexts such as chemistry. In particular, first year students struggled with ‘acids and bases’, both conceptually and in solving common problems. The primary confusion appeared to stem from a failure to recognise that the conventions of pharmacy differed from those familiar from chemistry, and a consequent attempt to use concepts and problem-solving approaches which had been applied successfully in chemistry but were inappropriate for the new context. Specifically, students focused on the physical characteristics of solutions of acids and bases, whereas the emphasis in pharmacy is on the structures which make a drug an acid or a base. While there are some situations in which concepts are directly transferable from chemistry to pharmacy, this is by no means universal, and students exhibited difficulties in discriminating between contexts.
In order to investigate possible reasons for these observations, we framed the problem as one of conceptual change, and drew on sociocultural approaches to learning to conduct research into both the processes and outcomes of conceptual change learning in a collaborative environment designed to facilitate the socialisation of students into their future profession of pharmacy. Using data collected during classroom interactions and individual interviews, we evaluated the extent to which students developed and used concepts which reflected the conventions of pharmacy.
Theoretical background
Sociocultural theories and conceptual change
Sociocultural theories, which are derived from the writings of Vygotsky, are based on the assumptions that learning and development are intrinsically social in nature and that individual processes originate in social practices (John-Steiner & Mahn, 1996; Rogoff, 1998). The fundamental tenet is that, as an individual participates in social practices, those practices become part of that individual’s repertoire through a process of appropriation. From this perspective, communication through language plays a fundamental role in learning, and individual learning is seen to be shaped by the specific social, cultural and historical contexts in which it takes place (Wertsch, 1991). The professional curricula of the contemporary university are therefore well suited to interpretation from a sociocultural perspective, through focusing on the ways in which the cultural practices (Miller & Goodnow, 1995) of a profession are learned by novices. For these novices, learning different cultural practices often involves conceptual 14change. Traditional conceptual change theories (Posner, Strike, Hewson & Gertzog, 1982) focus on replacement of incorrect concepts with correct alternatives, however a sociocultural approach is more concerned with the development of discrimination between different contexts and the ability to choose the concept which is situationally relevant (Driver, Asoko, Leach, Mortimer & Scott, 1994). Sociocultural theories also suggest that the idea of ‘concept’ can be broadened to include social and cultural practices (Säljö, 1999) and highlight the importance of participating in collaborative activity as a means of promoting change (Kelly & Green, 1998).
Collaborative interaction in an educational setting can facilitate enculturation into professional practice, particularly if authentic language and resources are used by participants engaging with realistic situations and issues. Learning occurs as individuals work collaboratively, often with the assistance of more capable guides, so that all are able to develop beyond their current capabilities, thus creating what Vygotsky (1978) termed zones of proximal development. Collaboration is regarded as something more than simply working together, in that it involves the development of intersubjectivity (Rogoff, 1998). In this chapter we describe how first year pharmacy students experienced conceptual change in relation to ‘acids and bases’ by learning to differentiate between language use and problem-solving approaches appropriate to chemistry and those appropriate to pharmacy, through engaging in groupwork within a weekly workshop during one semester. Through collaborative participation in specific learning activities, students learned ways of participating more successfully in the cultural practices of the pharmacy profession.
Communities, cultural practices and zones of proximal development (ZPDs)
The profession of pharmacy is an example of a community of practice (Wenger, 2000), which is a group engaged in particular cultural practices that come over time to be regarded as the property of the group and to constitute part of the personal identity of a community member (Miller & Goodnow, 1995). A community develops its own historical traditions and sociocultural identity, together with shared beliefs, patterns of language, and ways of carrying out its constituent practices (Säljö, 1999). Becoming part of the community entails learning to participate in ways which are recognised as characteristic of the community, and in particular learning its ways of communicating (Lemke, 1990). Depending on the nature of the community, different modes of communication may be appropriate, however the ability to communicate meaningfully through language is central to most human activities. Some aspects of the language used by one community may be shared with others, but often the meanings vary between communities. Pharmacy, for example, uses terms such as ‘acid’, ‘drug’ and ‘poison’ to communicate very precise meanings which are different from the meanings non-pharmacists would normally recognise. Communication through language is, however, rarely a matter simply of knowing definitions and using an appropriate vocabulary; rather it is a social process in which a group of participants create and sustain relationships through the making and sharing of meaning.
Socialisation into a professional community is a gradual process, and commonly involves a mixture of classroom learning and an apprenticeship of some type. Within the classroom, a type of community is created (Brown, 1997; Walker, 2003), with its own characteristic means of communication and cultural practices, but these are often idiosyncratic. A classroom cannot therefore mirror a professional community, but can be a safe environment for inexperienced peers to engage in relevant professional 15practices. The assistance of professional practitioners, often as tutors, allows students to evaluate their learning and appropriate more of the characteristics of the professional community. These conditions promote the formation of ZPDs, which are environments within which individuals collaborate on activities which they cannot successfully complete alone (Newman, Griffin & Cole, 1989). ZPDs are characterised by collaborative activity where each individual takes some form of responsibility for personal and group learning (Brown, 1997). Learning professional cultural practices is enhanced when ZPDs arise: final year pharmacy students at the University of Sydney, for example, learn clinical decision-making in the context of supplying prescription medicines though problem-based learning under the guidance of practicing pharmacists. In the early years of the degree, ZPDs are created as students learn appropriate patterns of language use through talking to each other. The latter is critical because students already have their own ways of talking about pharmacy topics – such as ‘acids and bases’ – which are different from those of pharmacy. In first year, one critical aim is to assist students to learn ways of talking which are characteristic of pharmacy, rather than chemistry.
Conceptual change through changing language practices
Learning a new way of talking about what appears to be a familiar topic involves conceptual change. When novices encounter a situation in which familiar words convey quite different meanings, they often experience conceptual confusion because they do not recognise that any difference exists. We believe that this is at the heart of the difficulties experienced by pharmacy students with ‘acids and bases’. In chemistry, these words are associated with physical features such as pH, corrosiveness, taste and feel, whereas in pharmacy the meanings revolve around molecular structure and behaviour in various environments: these differences in conceptual meaning determine the interpretation of problems and approaches to solving them. As suggested earlier, a sociocultural approach to conceptual change is concerned with discrimination of concepts and practices between contexts; this discrimination extends to the use of language (Säljö, 1999), and conceptual change can include enculturation into the language used within a particular community. Participation in the community itself, or in a well-structured learning environment, is critical for the development of fluency and confidence in this new language use through provision of opportunities to participate in discussion with both peers and professional practitioners. In the research reported in this chapter, we structured the workshop activities such that discussion was encouraged among the peer groups both in the absence and presence of the tutor, who was a practicing pharmacist.
Conceptual change and thinking together
The provision of learning environments designed to encourage discussion is important but not sufficient to promote conceptual change, since change is critically dependent on the nature of the interactions within the environment. As suggested earlier, collaboration involves more than simply working with others; it requires a sharing by the participants of the meaning and goals of a joint activity, and a willingness to engage with the group and the activity which results in intersubjectivity (Rogoff, 1998). Intersubjectivity is characterised by the ability of groups to work within a common frame of reference and to share their thinking (Tudge & Rogoff, 1989). Evidence for shared thinking in the current research was sought in the social interactions within the 16groups, primarily the language patterns, but also group dynamics. We were particularly interested in the relationship between group interactions, the development of intersubjectivity, and evidence for conceptual change.
Research context and methodology
The present research aimed to examine aspects of the process of enculturation into the pharmacy community through observing small collaborative groups of learners. In this chapter we focus on the patterns of conceptual change observed within two small work groups of first year pharmacy students learning about ‘acids and bases’. Students self-selected into these groups within a large workshop class which met weekly for two hours over one semester. No specific instruction was given to students about working in groups since it was of research interest to observe the patterns of collaboration which students would choose to adopt.
The context was Introductory Pharmaceutical Science, a compulsory component of the four-year Bachelor of Pharmacy at the University of Sydney with an enrolment of over 200, and consisting of lectures and workshops, all taught face-to-face. Within the workshops, groups of four to six students carried out assigned tasks including discussion of concepts, reflective exercises, solution of problems, and joint construction of explanations.
Change was explored through observing the language patterns used by members of the two groups, both in workshop sessions and in interviews. Three workshop sessions were scheduled to be videotaped for each group (although one group requested not to be recorded on the third occasion) and each member took part in three interviews: immediately prior to commencement of the topic ‘acids and bases’; immediately after the topic; and five months later, at the start of the following academic year. Examples of the questions are included in the appendix. Insights into the processes of change were gained through specific questions in the interviews and analysis of the social functioning of the groups during workshop sessions.
As the research progressed, considerable differences were observed between the two groups. In one case, the members began using language patterns appropriate to pharmacy and became more skilled in applying relevant critical thinking and problem-solving approaches; these changes persisted for at least five months. We describe this group as the persistent-change group (PC). In the other case, although short-term learning was apparent, conceptual change in the form of different ways of talking was not maintained beyond the end of the teaching semester. We describe this group as the transient-change group (TC).
The persistent-change group (PC). In the persistent-change group, all six members were female and aged between 17 and 19 years. Four of the six were not native English speakers, and indicated that they spoke either Cantonese or Vietnamese at home. All were fluent in spoken English. The group consisted of a nucleus of three friends, who had worked together during the previous semester and three additional students who were acquaintances but not friends.
The transient-change group (TC). The transient-change group consisted of three females and two males, all aged between 17 and 19 years. One male indicated that he spoke Cantonese at home, however he was fluent in English. All other members were 17native English speakers. Members described themselves as close friends, and had arranged their timetables to enable them to work together in this workshop. All were residents of residential colleges on campus, and studied and socialised together.
Exploring change
Evidence of learning and conceptual change
Evidence of conceptual change learning was sought in comparisons between the three interviews held with each student, and in particular the extent to which any changes persisted beyond the end of the year. Initial interviews before commencement of the topic established that students shared a chemistry perspective of the topic; the second interviews, held immediately after the topic finished, indicated that all students had learned to articulate key concepts and solve problems in ways consistent with pharmacy conventions. During the third interviews, held after the university long vacation, students engaged in discussions similar to those of the second interview, and were asked to solve problems like those encountered during the previous semester. They were encouraged to articulate their thinking while solving the problems. The third interviews were regarded as providing evidence of the persistence of any conceptual change, in contrast to the second interviews which were indicative of short-term learning. The results discussed in this chapter were obtained from the third interviews.
Persistent–change group. Members of the PC were generally confident in their answers to questions about the characteristics of an acid, identification of acidic and basic functional groups, the association of pKa rather than pH1 with acidic drugs, and the meaning and function of pKa; all of these dimensions reflected relevant pharmacy conventions. Their explanations tended to be concise and came to the point without needing excessive prompting. Their language was generally consistent with pharmacy usage, and was reflective of group discussions during workshop sessions. Not surprisingly, they were unable to articulate all of the material learned in the previous semester, but they tended to have forgotten specific points, rather than confused concepts. The interviews tended to be short, as extensive probing was not required to stimulate articulation of their understanding.
Excerpts from the interviews serve to illustrate a number of critical findings.
Excerpt PC1
Veronica: Um, a drug is acidic because it has an acidic functional group on it…Acidic drugs have acidic functional groups on them but there’s other parts to the drug as well. Whereas acids are just acids itself.
One of the key differences between chemistry and pharmacy is definitional. Students are familiar from chemistry with physical properties of acids such as pH, feel and corrosiveness, whereas pharmacy conventionally focuses on molecular structure. Veronica clearly and concisely articulates the pharmacy convention using language appropriate to the context and indicates that she is able to discriminate between prior learning and her new conceptual understanding.
18
Excerpt PC2
Isabelle: That is an acidic group.
Interviewer: Yes.
Isabelle: And I can’t see anything else so I would call that an acidic drug.
Isabelle also demonstrates an appreciation of the importance of molecular structure, and confidently identifies the sole functional group correctly, then uses this identification to classify the molecule correctly, using appropriate language.
Excerpt PC3
Interviewer: Do you think of acids as having a pH?
Kellie: No, they have a pKa. I think pH is for solutions that contain acids and bases.
One of the key issues identified in previous observations of conceptual difficulties is the association of acids and bases with pH. pH is often associated in chemistry with acids and bases, although it is considered in pharmacy as the property of a solution of an acid or a base. When acids are identified on the basis of molecular structure as they are in pharmacy, the significant parameter is pKa rather than pH. The acknowledgement by students of the importance of pKa rather than pH in the context of pharmacy is key evidence for conceptual change learning.
Excerpt PC4
Denise: Ka is the acid dissociation constant. Which is like the products over the reactants. And then pKa is minus log of the Ka.
Interviewer: What do we use pKas for? What do they tell us?
Denise: The extent of dissociation.
Denise also clearly demonstrates pharmacy conventions and definitions. Although pKa is also important in chemistry, students generally give it less importance than pH, thus familiarity with the meaning of pKa is important as evidence for learning.
Transient-change group. The students in the TC were considerably less confident than those in the PC. Their responses were typically a mixture of pharmacy and non-pharmacy conventions, and on many occasions appeared to reflect a combination of pre- and post-instruction responses. In contrast to the PC, several TC students relied on memorisation of the material, and stated explicitly that they needed to write the answers down rather than articulate them verbally. Interviews tended to be longer, as substantially more prompting and probing was necessary, and the interviewer frequently resorted to leading questions in an effort to encourage verbal responses. Several of the students were capable of solving the problems presented to them, with assistance, but their solutions tended to be idiosyncratic rather than according to pharmacy conventions. 19
Excerpt TC1
Larry: Well, as you said, drugs tend to be not as acidic, they’re more… You don’t, yeah… There is a difference between, like most acidic drugs don’t tend to be as strong as normal acids. Like your Hydrogen Chloride or whatever is a much stronger acid, whereas your acidic drugs are not as strong.
Interviewer: What’s the difference? What kind of characteristics, apart from strength, might be different between say, hydrochloric acid and a drug which is an acidic drug?
Larry: They contain weaker acidic groups. Like carboxylic acid.
Interviewer: What do you mean by strength, then? What does a strong acid do that a weak acid doesn’t?
Larry: A strong acid dissociates completely, or it’s meant to. Most acidic drugs don’t dissociate completely.
Larry was clearly lower in confidence as evidenced by his use of incomplete sentences and long pauses. His replies demonstrate conceptual confusion, involving a mixture of chemistry and pharmacy concepts, with little apparent discrimination between contexts. His responses focus primarily on strength rather than molecular structure, despite probing for alternative concepts. In addition, strength is explained using chemistry conventions rather than pharmacy: in pharmacy strong acids are defined in terms of low pKa rather than complete dissociation.
Excerpt TC2
Lucy: pH and Ka but I just can’t remember the relationship between it. That’s terrible it’s only been, it hasn’t been very long.
Interviewer: OK. Does it have anything, do you associate pKa with strength of acids and bases?
Lucy: Yeah, but I can’t remember which one it is, whether it’s high or low.
Interviewer: So pKa has something to do with strength but you would need to re-memorise which one went with which.
Lucy: Yes.
Lucy was characteristic of her group in that she tended to rely on recalling aspects which she had studied for the exam, rather than demonstrating evidence of conceptual understanding. In this case her memory fails her. Another member, Janine, also indicated that memory was important in her learning and commented that memorisation was one of her preferred study methods. Janine was, however, able to remember considerably more than Lucy.
Excerpt TC3
Interviewer: What makes something an acid? 20
Geoffrey: Donates a proton.
Interviewer: Anything else that you think of as being characteristic?
Geoffrey: Well, you mean physical characteristics? Taste and stuff, for example? Yeah, I suppose.
Interviewer: You suppose?
Geoffrey: Yeah, I’ve just stuck with those since Year 8 chemistry. Tastes sour, all that sort of stuff.
Geoffrey’s explanation is based on pre-instruction concepts, and uses chemistry conventions. The role of secondary school chemistry is evident in shaping his conceptual understanding, and his ideas had apparently not developed from a consideration of physical characteristics (chemistry) to the alternative of molecular structures (pharmacy).
Excerpt TC4
Larry: That is a thingy group, amine, did you call it?
Interviewer: So what does that make lignocaine?
Larry: That makes it a acidic drug.
Interviewer: Nope.
Larry: It’s a basic drug?
Interviewer: Amines are basic.
Larry: Does phenols make it acidic?
Interviewer: Phenols are acidic, yes.
Larry: Ah, see, there you go. Assume the OH group. OH groups are basic, normally.
Larry’s response demonstrates conceptual confusion in the identification of functional groups, suggesting the persistence of chemistry conventions. Phenols are weakly acidic molecules with an OH group directly attached to a benzene ring, but Larry retains a concept of OH as basic, confusing it with the concept of hydroxide ions (OH-). His terminology is also imprecise, again suggesting a lack of conceptual clarity.
As these short excerpts suggest, the two groups differed significantly in the persistence of conceptual change. Five months after learning the material, PC members remained able to articulate pharmacy language clearly, concisely and confidently and were able to discriminate between chemistry and pharmacy concepts. On the other hand, TC members showed evidence of regression to prior conceptual understanding and a blending of ideas with little acknowledgement of the differences between the two contexts. TC members were less confident in using pharmacy language, and tended to rely on memory rather than a long-term change in understanding. Explanations for the 21observed differences in conceptual change persistence were sought in an evaluation of the social functioning of the two groups during workshops. Results from interviews and brief observations from the workshop sessions are presented in the following section. It is clear that the two groups operated in substantially different ways.
Social functioning
Persistent-change group. All members of the PC commented favourably on the experience of working within this group during the semester. Each felt comfortable within the group, and indicated that other members were supportive, cooperative and helpful. They felt the group was cohesive, and that all contributions were valued. Expressing ignorance or difficulty in understanding was encouraged, both as an opportunity for another member to offer an explanation (and enhance their own learning in the process), and as a rationale for requesting assistance from a tutor. There was little evidence of competition between students, rather a strong sense of collaboration whereby success involved learning for all members. Friendships were created and strengthened, and all students indicated that they would be happy to work with all other members of the group again. The following excerpts serve as illustrations.
Denise: Oh I think, like we all worked really well. Because yeah they were really nice people and they were easy to work with. Yeah they were all really cooperative.
Kellie: I think we worked well together. ’Cause we all shared ideas and tried to work through the problems together… It felt like, we were all there to help each other. And help each other learn. So it was no problem if you didn’t understand something. You would just speak up and then someone would try to explain it. And then it helps them as well. They learn from it as well.
Isabelle: They’re friends now, they’re my friends now and I just, they’re easy to talk to and you don’t feel, like even though I felt less intelligent I still didn’t feel bad when they told me, hinted answers or whatever, so they were good to get along with and understand things.
Observation of this group during workshops supported these self-reports, and revealed that the group used a number of collaborative strategies to facilitate their learning, including a focus on participation, sharing of leadership within the group, mutual respect for other members’ contributions, and listening and responding constructively. Group processes were characterised by inquiry, involvement of the whole group in solving a problem, and willingness to try out pharmacy language. These strategies, characteristics and processes provided evidence for the development of high levels of intersubjectivity, and the creation of ZPDs which allowed members to achieve beyond their individual capabilities.
Transient-change group. The TC began as a group of close friends, but as the semester progressed, tensions became apparent. One member was perceived as operating primarily for herself, and behaving in a manner which discouraged the other members. This student was perceived as particularly conscientious in her study habits, which distinguished her from the remaining members. Group work was seen as having the potential to hold back such students, who were often faster at solving problems. This observation suggested that at least some members of the group regarded completion of 22the tasks – rather than learning – as the primary purpose of their participation. Friendships were strained rather than strengthened as a more competitive atmosphere prevailed.
Geoffrey: Sometimes it’s better if you work by yourself though, like sometimes it can be, it might slow you down a bit which is I think what Janine sees the group work as. Like sometimes she might see it as slowing her down a bit because she knows what she’s doing and she sees it more as, rather than helping us I think she sees it more as an interruption to her work.
Geoffrey: We’re pretty much a group of friends except um not so much with Janine lately though. I think I’m speaking on behalf on everyone because when she goes into study mode she can be very, um, she comes across as, my opinion is that she comes across as being very, very arrogant sometimes.
Larry: And we had Janine there, who’s not exactly helpful, when she, when the rest of us are trying to do it at her pace. She works at a much quicker pace. She does so much more study than we do, so she knows, she comes in there, she knows what to do, she does it, and when you try to get help from Janine, or, you know, you try to do anything with Janine, she just somehow just makes you feel almost stupid …. It’s hard to work with her in a group, because if you’re not up to her level, then she doesn’t appreciate the fact that you’re trying to learn it.
Lucy: Geoffrey or Larry were usually the best at helping me cause, I don’t know, I’m closer to them I think personally than the other two, Janine and Emma, so and I think that’s just it really. I get along better with those two so that helps me understand.
Observation of this group also supported their individual reflections. Members rarely worked in more than pairs, and several students clearly preferred to work individually and at their own pace. Assistance tended to be sought from a tutor rather than another group member, and questions usually originated from one or two students, rather than from a whole group consensus (which was common in the PC). As a result, discussion of the material was limited, and extended exchanges in the context of problem-solving were far less frequent than in the PC. The discussion which did take place tended to be of a peer-teaching nature, with one student giving the answer to a problem, or stating an approach to follow, rather than a more collaborative exploration by all members. Consequently, the extent to which members of this group practised pharmacy language was significantly less than that for the PC. Intersubjectivity levels were low for most of the workshop sessions, as students did not share their thinking to a great extent, and ZPDs were rarely evident.
Discussion and conclusions
The evidence from the investigation presented in this chapter is consistent with the argument that conceptual change learning is promoted by collaborative activity which results in intersubjectivity, and the development of ZPDs. In particular, the extent and nature of group discussion was central to the persistence of learning beyond the immediate teaching period. The PC engaged in social interactions which supported 23discussion and exploration of difficulties in understanding, and were able to develop shared thinking by expressing their ideas in a supportive atmosphere. Their shared discussion, or social knowledge, was then able to be appropriated into the individual’s conceptual understanding, which persisted for a substantial period after formal classes. On the other hand, the TC engaged in more individualistic and competitive behaviours which did not promote intersubjectivity or the creation of ZPDs. Members of the TC did not engage in extended discussion and thus experienced reduced opportunities for appropriating social knowledge. A consequence their individual conceptual understanding did not develop to the same degree as the PC. In particular, members of the TC exhibited confusion between contexts which was not evidence in the PC, and a significantly lower ability to communicate appropriately in the context of pharmacy.
Our findings are consistent with those of Barron (2003) who identified patterns of collaboration which differentiated groups which were more successful in solving problems from groups which were less so. She found that successful groups were far more likely to engage with a problem, and to demonstrate sensitivity towards each other, awareness of each other’s progress and a willingness both to contribute and to listen. Within the less successful groups, competitive interactions and individual attempts at problem-solving hindered joint activities and the likelihood of achieving a solution. These patterns were evident in the two groups in our research, and similar relationships were observed between collaboration/competition and success, where success was regarded as persistence of conceptual change learning. Palincsar, Anderson and David (1993) identified four social patterns of activity which were associated with successful collaboration: contributing to the group, giving reasons for suggestions, making efforts to understand, and building on other students’ ideas. Our results were also consistent with these observations in that productive patterns were strongly evident in the interactions of the PC but only minimally in the TC. It is interesting to note that members of the TC actually outperformed members of the PC by approximately ten percentage points in the end-of-unit examination, suggesting that the TC was not academically inferior. We have represented the relationships between social functioning and learning processes and outcomes in Figure 2.1.
Barron (2003) further identified personal relationship or friendship as a critical aspect of group learning. Her review of the literature suggested that interactions between friends were more productive than interactions between individuals who were not friends. Our results suggest that the impact of friendship on collaborative learning is more complex. Members of the PC strengthened their friendships throughout the course of the workshops, whereas the relationships within the TC deteriorated. Many interrelated factors were responsible for these contrasting outcomes, and the area is one in which further research would be illuminating.
It is clearly difficult to extrapolate research findings from two small groups within a much larger cohort to the wider population, but continuing analysis of the data presented briefly in this chapter is currently illuminating more details about patterns of activity and interaction which appear to promote learning, and patterns which appear to be less productive. Exploration of these patterns should provide guidance to teachers 24and students about how to enhance learning through collaboration. Nevertheless, there are a number of implications which can be drawn to this point, including the importance of collaboration in groupwork and of providing opportunities for students to discuss their understanding and approaches to problem-solving. Learning activities are ideally structured so that ZPDs can form; this suggests that the activities should be slightly more difficult than students’ current capabilities but not so difficult that they cannot engage with the activity at all. This research did not explore the impact of how groups are created, however it is likely that issues which arise for self-selected groups may differ from issues associated with allocated groups and this avenue is worth pursuing in this context. Finally, although a minor aspect of the current research, the finding that persistent conceptual change learning was not obviously related to examination performance poses challenges for all academics seeking to ensure that learning is appropriately encouraged, acknowledged and recognised. The final examination in Introductory Pharmaceutical Science was a written paper designed primarily to assess skills in problem-solving, both numerical and word-based, thus provided little opportunity for students to demonstrate spoken language patterns. Assessing the latter is problematic, particularly for large cohorts, and while a number of approaches have been suggested (for example Magnusson, Templin & Boyle, 1997; Sainsbury & Walker, in press), considerable challenges still exist in designing assessments which are both authentic and practicable.
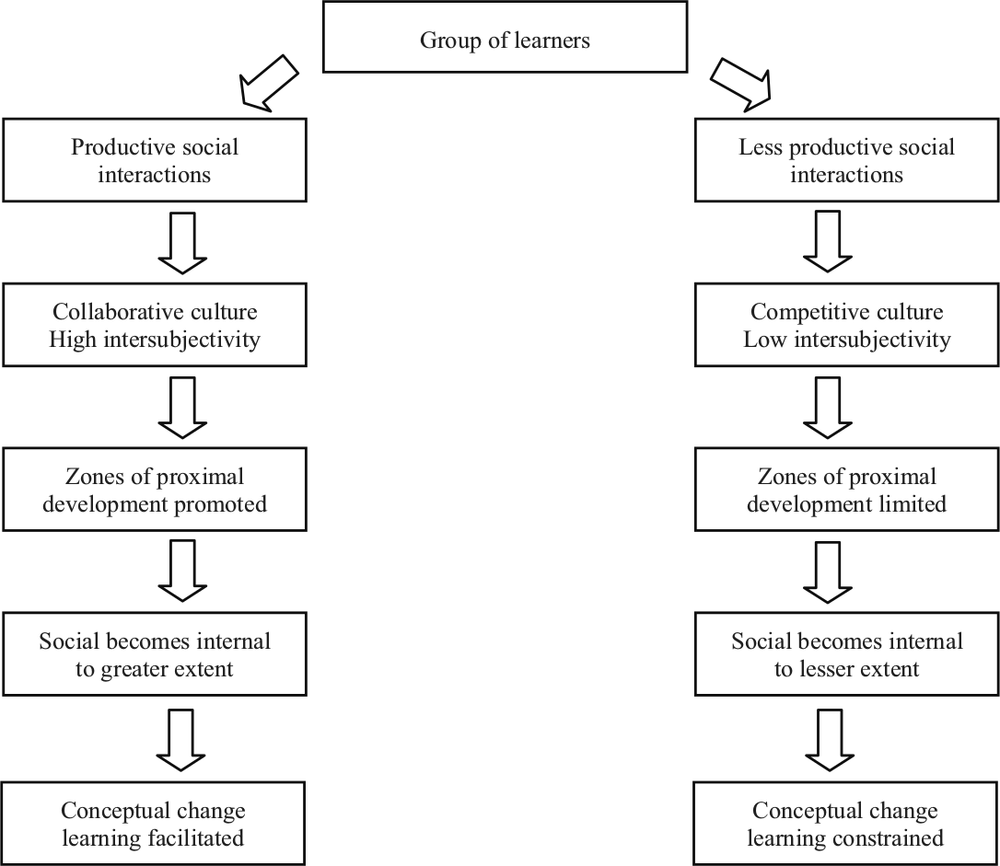
Figure 2.1. Group culture and conceptual change
Changes in practice
The findings from this research have been implemented in a number of ways. Firstly, although some specific indications were given to study participants about the 25differences between chemistry and pharmacy terminology, it was clear that more explicit assistance would be beneficial, and this has been implemented in subsequent teaching. Secondly, the findings have been discussed with students and the purposes behind working in collaborative groups have been more clearly outlined. Thirdly, more attention has been focused on assisting students to learn how to work productively in groups. This assistance is incorporated into a unit of study earlier in first year which is designed to facilitate both socialisation into the profession of pharmacy and transition to tertiary study. Fourthly, the collaborative workshop approach has been adopted by a number of colleagues responsible for a number of other units of study, as a means of facilitating learning more broadly within the Faculty.
Appendix: Examples of interview questions
Each interview was semi-structured and was responsive to the student’s comments, however each covered three primary questions, namely:
- What makes something an acid or a base? What are the characteristics of an acid or base?
- What is meant by the strength of an acid or base?
- Does acid always mean the same thing to you?
The depth or breadth of discussion of each question was unique to each interview. Students were also asked to engage in a number of problem-solving activities, while verbalising their thinking and reasoning. These activities included:
- Given these structures, can you identify the functional groups and tell me if the molecule is an acid or base or something else?
- Can you tell me pH values where these drugs would be completely ionised and completely unionised?
Depending on the student’s response, items of more complexity were also added on occasion. 26
1 pH and pKa are two parameters associated with acids and bases. pH refers to the concentration of hydrogen ions in a solution containing acids and/or bases, whereas pKa is a reflection of the equilibrium constant for the dissociation of the acid or base in water. In chemistry, the focus is more on pH; in pharmacy the focus is on pKa as a characteristic of acids and bases.