20
Managing diabetes complications in the clinical arena
In the absence of a complete prevention or cure for diabetes mellitus in all its forms, complications will occur in most people with diabetes. These are common and varied. The spectrum includes psychosocial as well as biological complications and they may be acute, as in those causing very low or high blood glucose. Chronic biological end-organ complications of diabetes include so-called microvascular complications of diabetic eye, kidney and nerve disease and the macrovascular complications of cardiovascular, cerebrovascular and peripheral vascular disease. Evidence from clinical trials indicates that using current healthcare and therapy standards, much can be done to prevent onset and progression of diabetes complications; indeed, over recent years, serial reductions in death rates in people with diabetes reflect such beneficial outcomes. This chapter will focus on trends and options in care as well as therapies that hold promise to improve complications outcomes in people with diabetes.
The spectrum of diabetes complications
Diabetes mellitus is essentially a syndrome where the hormone insulin is deficient in the body. As a result there is not enough insulin to prevent increases in blood glucose, or to normally regulate protein and body fat [1]. While blood glucose levels may be quite mildly elevated at certain times in some people with diabetes, if insulin is severely lacking it can rapidly lead to emergency, life-threatening conditions of diabetic ketoacidosis, where acid in the blood occurs, or hyperosmolar coma where blood glucose levels rise to very high levels and may cause coma [2].
A major breakthrough occurred in diabetes care when insulin was discovered as the hormone produced by the pancreas which is deficient in diabetes. A short time after that discovery in the early 1920s, people with diabetes, especially young children, had their lives saved by insulin injection treatment and emergency conditions such as diabetic ketoacidosis (DKA) were largely avoided, and quality of life was significantly improved [3]. The source of the insulin was initially isolated from animal pancreas, and was subsequently derived from human insulin made in the laboratory. However, after the discovery of insulin
354and its lifesaving role in therapy, it became increasingly clear over the subsequent years and decades that people with diabetes were at increased risk of developing a series of different complications from diabetes [4], namely damage to certain organs and tissues, especially the eyes, kidneys, nerves, heart and blood vessels. These complications are listed in Table 1. Research into the cause of diabetes complications and the mechanisms to prevent, detect and treat them, is the main focus of this chapter.
Table 1. The spectrum of diabetes complications, including acute and chronic, organic and psychosocial.
| Time course | Complication grouping | Specific complications |
|
Acute (usually hours to days) |
Insulin lack | diabetic ketoacidosis, hyperosmolar nonketotic coma |
| Insulin excess | Hypoglycaemia | |
|
Chronic (usually years) |
Microvascular | Diabetic retinopathy, diabetic nephropathy, diabetic neuropathy |
| Macrovascular | Cardiovascular disease, cerebrovascular disease, peripheral arterial disease | |
| Other (see text for detailed list) | Including congenital malformations | |
| Psychsocial | General | Depression, eating disorder |
| Diabetes specific | Diabetes distress |
Acute complications of diabetes
(i) Acute complications due to a lack of insulin
Amongst the three main types of diabetes mellitus – type 1, type 2, and gestational diabetes – the former two in the absence of a pancreas transplant, are incurable and persist, whereas the latter condition is transient. In type 1 diabetes there is absolute insulin deficiency. This means that in the absence of insulin therapy, cells in the body will become starved of glucose. Insulin is an important anabolic, or building-up, hormone that helps blood glucose move into tissues, and cells to create cellular energy, and it prevents breakdown of body fats and muscle. Insulin acts like a key to help glucose move into cells. In the complete absence of insulin, the cells in the body, especially the liver and skeletal muscle, cannot ‘see’ glucose outside the cell. As a result, the alternative form of stored glucose, known as glycogen, is rapidly broken down by fat and muscle. However glycogen is completely used up within 24 hours. The fat and muscle in the body are then broken down and the liver takes up fatty acids from the circulation to make an alternative form of energy for the body known as keto acids. While these acids, also known as ketone bodies, provide 355a source of energy to cells, over subsequent hours and days in the absence of any insulin the production of keto acids increases to severe levels [2]. The tissues and the circulation become progressively acidic, placing the defenses against pH imbalance at these sites under great physiologic stress. If the blood glucose is high, the blood is acidic and the blood or urine ketones are elevated, then a person is said to have ‘diabetic ketoacidosis’ (DKA). A person who develops DKA, will become ‘hot and dry’; he or she will increasingly become dehydrated as large volumes of urine are passed due to elevated blood glucose, and will often be febrile due to having acid in the blood, causing an elevated body temperature. The person with DKA also attempts to breathe off carbon dioxide (CO2) which is formed to excess in the body when CO2 is produced from the alkali bicarbonate as a buffer combining with the excessive blood acid from ketones.
DKA is a medical emergency. If it is detected early enough, it can be readily treated with intravenous fluid and insulin and restoration of the body’s ions, especially potassium, all with close monitoring [5]. Each year in Australia, some people with type 1 diabetes die due to diabetic ketoacidosis [6]. The risk of DKA in established type 1 diabetes is 1%–10% per patient per year [7]. The risk of DKA is increased in people with poor glycaemic control or previous episodes of DKA; peripubertal and adolescent girls; children with psychiatric disorders, including those with eating disorders; children with difficult or unstable family circumstances; children with limited access to medical services; and people who omit insulin [7]. Any death due to DKA is a disaster. If treatment is provided early enough, mortality should not occur, especially in people who have known type 1 diabetes. Increasingly, guidelines to help prevent DKA and to detect this acute complication early are being developed [8].
If a person with type 2 diabetes becomes severely deficient in insulin, the blood glucose levels may become markedly elevated over subsequent days and weeks [9]. For example, some people may develop levels of blood glucose above 50 mmol/L, compared with the normal non-diabetic range of about four to eight mmol/L. As blood glucose levels become elevated the glucose is lost into the urine, taking water with it and causing dehydration. The very high blood glucose levels affect consciousness and can cause confusion, and loss of consciousness or coma. This condition known as ‘hyperosmolar non-ketotic coma’, (or HONC), is life-threatening. It requires emergency room treatment and intensive care support. With careful rehydration and infusion of insulin, as well as treatment of the factor that may have destabilised diabetes, such as an infection, most people with HONC will survive.
Why people with type 2 diabetes may develop HONC, and yet people with type 1 diabetes can develop DKA, is an important clinical observation that has not been fully explained. In brief, it appears that in HONC, the body makes enough insulin to prevent keto acid formation but not enough to prevent blood glucose rising excessively, whereas the absolute insulin lack in type 1 diabetes leads to DKA. It has been observed repeatedly however that if some people with type 2 diabetes become severely acutely unwell, for example from a severe infection in the body or due to a heart attack (myocardial infarct), then DKA may transiently develop or they may develop a mix of HONC and DKA [9]. Distinguishing 356between type 1 and type 2 diabetes can be difficult and it is increasingly being recognised that some people with apparent type 2 diabetes, on careful assessment or in the course of time, develop a picture of type 1 diabetes [8].
(ii) Acute complications due to an excess of insulin
If blood glucose levels fall below the normal range, causing hypoglycaemia, it can make a person with diabetes feel unwell, with sweating and tremor and other symptoms, and in the most severe situation, loss of consciousness, known as ‘hypoglycaemia coma’. Severe hypoglycaemia may be life-threatening, for example, by causing seizure, injury or precipitating heart attack or abnormal heart rhythm events [8, 10]. Essentially, hypoglycaemia occurs because treatments used for diabetes, either insulin or medications that cause increased insulin release from the pancreas, lower blood glucose excessively for the needs of the body at a certain point in time [11].
Hypoglycaemia of all forms is, understandably, feared by people with diabetes and their immediate carers and family [12]. Fear of hypoglycaemia and its consequences, including night-time episodes, is a major barrier to optimal blood glucose control in type 1 diabetes and in type 2 diabetes when insulin treatment is required [13]. For most people with type 1 diabetes, mild (ie self-treated) hypoglycaemia remains a regular occurrence. It may occur about twice weekly [14] and adversely affect quality of life. In contrast, severe hypoglycaemia occurs on average once every three or more years in type 1 diabetes [11, 14]. There is marked individual variation in the rate of severe hypoglycaemia. Some people never experience it, whereas in others it occurs multiple times a year, despite intensive diabetes support in management [11]. Nocturnal hypoglycaemia accounts for close to half of the episodes of severe hypoglycaemia in type 1 diabetes [14]. In type 2 diabetes, for people on insulin therapy, mild hypoglycaemia occurs in about 30% of people each year, and severe hypoglycaemia in 1%–2% [15]. Risk factors for severe hypoglycaemia in type 1 and type 2 diabetes include a long duration of diabetes and aiming for tighter blood glucose control [8, 11, 15].
Prevention of hypoglycaemia is an important aspect of diabetes care. In people with type 1 diabetes or type 2 diabetes on therapy that may lower blood glucose such as insulin, balancing blood glucose across the day by considering carbohydrate intake, physical activity and insulin dosage is a constant consideration [8, 11]. Some forms of insulin therapy and diabetes management regimens can help to prevent mild and severe hypoglycaemia. So too can insulin pump therapy, especially those that combine state of the art technology with real-time continuous blood glucose monitors [8].
Treatment of mild hypoglycaemia by oral glucose is usually highly effective, taking 15 to 20 minutes to begin to have effect [11]. In severe hypoglycaemia causing unconscious, intravenous treatment with glucose by a paramedic or a medical doctor, is usually effective. Alternatively, a hormone therapy known as glucagon, which mobilises glucose into the blood stream from glycogen stores in the liver, can be administered by intramuscular injection by a carer who has been educated in the administration, or by a health professional [13].357
Table 2. Differing perspectives on diabetes complications.
| More negative perspectives | More positive perspectives |
| Diabetes end-organ complications occur in most people with diabetes | Most diabetes complications that occur are mild to moderate in severity and complications can often be detected early and their progression prevented |
| Due to its high prevalence in Australia diabetes is the single commonest cause of kidney failure, and also working age blindness | Less than 2% of people with diabetes will develop end-stage kidney disease or blindness annually |
| More than 15% of people with diabetes will develop at least one foot ulcer in their life-time | The vast majority of foot ulcers that occur in people with diabetes will heal with proper care without requiring extensive amputation |
| The commonest cause of death in people with diabetes is cardiovascular disease | Yearly rates of death due to cardiovascular disease in people with diabetes have been falling by more than 50% in developed countries over recent decades, due likely to multiple improvements in patient care including blood cholesterol, blood pressure and blood glucose control |
| Routine screening for some diabetes complications on a regular basis is not yet established for some conditions such as diabetic cardiomyopathy or liver disease related to diabetes | Screening for some diabetes complications such as diabetic retinopathy, nephropathy and neuropathy and foot disease are well established and can help to prevent severe complications |
| Hypoglycaemia is common in people with diabetes and is due to doses of certain medications such as insulin causing an excessive lowering of blood glucose | While severe hypoglycaemia is ten to 20 times more common in those with type 1 compared with type 2 diabetes, most hypoglycaemia that occurs in all types of diabetes is mild, and severe hypoglycaemia can be prevented |
Chronic diabetes complications
Diabetes complications can be divided into those affecting the small vessels of the body, so-called microvascular, and those affecting the larger vessels termed macrovascular. Microvascular complications are mainly those affecting eyes (diabetic retinopathy), kidneys (diabetic nephropathy) and nerves (diabetic neuropathy). Macrovascular complications are those of the heart (cardiovascular disease), brain vessels (cerebrovascular disease) and the limbs (peripheral arterial disease). There are other diabetes complications which may be mixed in type and are discussed subsequently.
In 2009, diabetes was among the top ten leading causes of death, being the direct cause of 3% of deaths in Australia, and contributing to another 7.1% of deaths [16]. In addition, the presence of diabetes complications increases the financial cost of diabetes at least twofold [10]. Diabetes is over-represented as a cause of death in the Indigenous population, where it was responsible for 8% of all Indigenous deaths, compared with 2.9% of deaths in non-Indigenous 358people [16]. Probably due mainly to the increased prevalence of diabetes in the Australian community, the total number of deaths to which diabetes has contributed has progressively increased over the last 20 years [10, 16].
(i) Pathogenesis of diabetes complications
Why some organs, and tissues in them, are more susceptible to injury than others is not clear and is the subject of ongoing intensive research [17]. However, there are some common elements amongst the differing body parts that develop diabetes complications. In body sites affected by elevated blood glucose, tissues may become inflamed, or they may develop fibrosis/scar tissue or both [18]. Figure 1 indicates a schematic implicating biochemical pathways and adverse tissue changes that occur in diabetes complications. In inflammation, white cells are attracted into the tissue and inflammatory fluid may accumulate. The inflammation is persistent and of low intensity. In other cases the native cells in the tissue die and are replaced by scar tissue, also known as fibrosis, which involves new blood vessel growth and lay down of proteins termed extracellular matrix (ECM). Amongst the organs and tissues affected by diabetes complications, some may predominantly develop inflammation, some fibrosis, and others a mixture of the two. For example in diabetic nephropathy, there is evidence that inflammation in the kidney then leads to fibrosis with loss of kidney function [19].
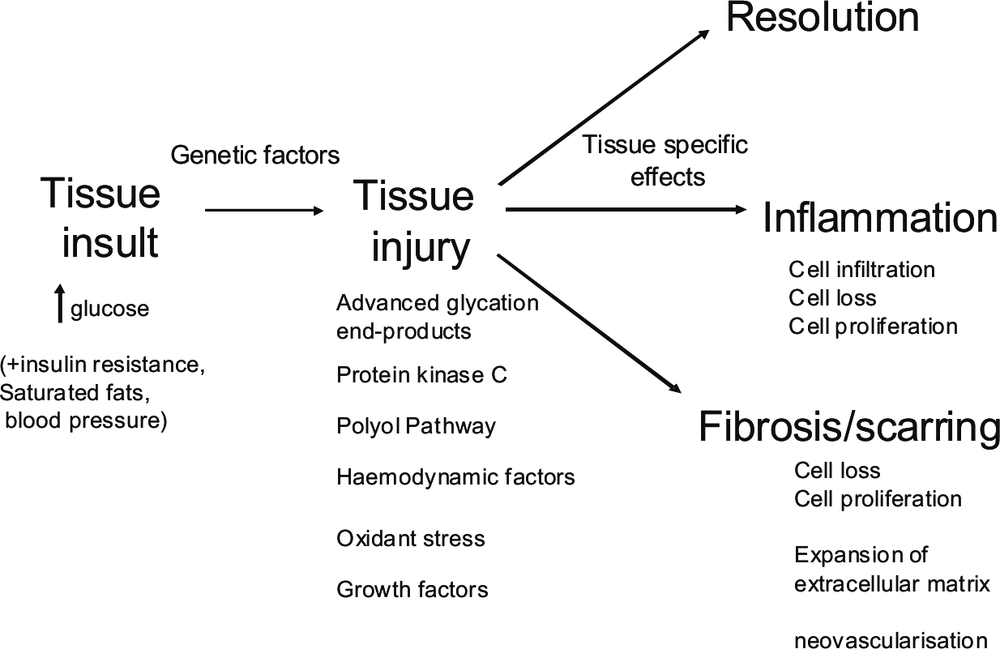
Figure 1. Schematic showing pathogenesis of diabetes complications based on biochemical and other cellular pathways activated (tissue injury), and tissue pathological change, which may lead to functional loss. High glucose interacts with genetic factors to cause tissue injury, tissue cell loss and in some cases, loss of function in a tissue. For further explanation refer to the text.
359How elevated blood glucose in diabetes leads to diabetes complications is under intensive research [20, 21]. In brief, from a biochemical perspective, high glucose in the blood will ultimately diffuse into cells and cause elevated cell glucose. The main biochemical pathway that normally handles glucose, known as the glycolytic pathway, and the subsequent biochemical pathways known as the TCA cycle and oxidative phosphorylation, become overwhelmed by the excess glucose being metabolised and forced into it. As a result, a number of changes occur in the biochemistry of cells. The high activity in the oxidative phosphorylation pathway leads to dysfunction in the main energy forming part of the cell known as the mitochondria and to formation of excessive oxidative stress in cells. In addition, a number of overflow metabolic pathways that are not usually very active, become much more active as metabolites in the glycolytic pathway accumulate downstream of glucose. These overflow pathways include those termed the polyol pathway, the advanced glycation end-product pathway, the hexosamine pathway, and the diacylglycerol-protein kinase C related pathway [20]. Other factors that may mediate adverse effects of elevated glucose in cells and tissues are proteins known as growth factors and cytokines. For example, the growth factor known as connective tissue growth factor is induced by all of the glucose overflow pathways, and it can cause both inflammation and fibrosis in tissues affected by diabetes [18, 19]. Alterations in matrix degradation, in particular by matrix metalloproteinases and their regulators, also contribute to tissue damage in diabetes [22, 23, 24].
Despite the above knowledge, the development of diabetes complications remains somewhat of an enigma. Diabetes complications to some degree occur in practically every person who develops diabetes, although severe complications occur in only a minority [8]. For example, elevated blood and tissue glucose is only one important risk factor for diabetes complications. Amongst the microvascular complications, identical twin studies have shown that genetic factors account for one-third to one-half the variation in diabetes complications, and there are likely both protective genetic factors as well as susceptibility factors [25]. To date only some of these genetic factors have been identified. It is thought that the interaction between genetic susceptibility with the metabolic effects of elevated blood glucose causes the tissue complications of diabetes [21, 25]. It is notable that, while increasingly elevated blood glucose levels increase the risk of diabetes complications, some people with diabetes can have quite poor blood glucose levels long term and yet they develop minimal or no damage to tissues, whereas, in others, mildly elevated blood glucose can be associated with severe organ complications.
In addition to elevated glucose, there are other factors that can affect diabetes complications risk. Elevated fats, especially saturated fats, and abnormal cholesterol levels with low levels of ‘good cholesterol’ (termed high-density lipoprotein cholesterol or HDL-C) and elevated and reduced quality ‘bad cholesterol’ (termed low-density lipoprotein cholesterol or LDL-C), contribute especially to macrovascular complications risk [26]. Haemodynamic factors, especially elevated general body (systemic) blood pressure contribute to both micro- and macrovascular complications risk [26, 27]. Increased waist circumference and central body fat are associated with high blood pressure, abnormal fats and also resistance to the action of insulin. In people who have these characteristic features of insulin resistance, abnormal blood fats and cholesterol, and high blood pressure, the ‘insulin resistance’ or ‘metabolic 360syndrome’ is said to be present. Presence of the metabolic syndrome increases the risk and severity of complications in people with type 1 diabetes [27, 28].
In pregnancy, women who have pre-existing diabetes (type 1 or type 2) are at increased risk of having a pregnancy complicated by miscarriage, or certain birth defects, or having large babies due to effects of excess blood glucose on the developing fetus [29, 30]. In contrast, gestational diabetes, or GDM, develops in later pregnancy (usually 24+ weeks of gestation) and likely occurs due to metabolic effects of the placenta and pregnancy placing metabolic pressure on the mother’s pancreas. GDM is usually a temporary form of diabetes and the elevated blood glucose tendency resolves after delivery. In pregnant women where the pancreas cannot make more insulin to overcome insulin resistance of the pregnancy, GDM develops. GDM increases the risk, in particular, of large babies at birth, leading potentially to obstetric complications at birth, stillbirth, and also increased risk of metabolic syndrome, overweight and obesity, and diabetes in later life [31, 32]. GDM also increases the risk of development of type 2 diabetes in the mother over subsequent years [32].
Research into diabetes complications occurs at the clinical and pre-clinical levels. As end-organ diabetes complications take many years to develop in humans, animal studies and cell-based research in the laboratory can help to examine diabetes complications in a more timely manner and to test differing interventions to prevent and treat diabetes complications [33]. The preclinical studies thus have advantages, although each is only a model of human diabetes and its complications, and are what is termed ‘hypothesis generating’. Ultimately human clinical studies are required to determine best evidence methods to manage diabetes complications.
(ii) Specific organ diabetes complications described
A. Microvascular complications
Diabetic retinopathy
In the course of time, most people with diabetes will develop some form of change to the back of the eye (retina) [34]. The most common and non-vision-threatening type of diabetic retinopathy is termed ‘non-proliferative’, where neither growth of new vessels or severe leakiness of the retina is a concern. There are two vision-threatening types of diabetic retinopathy. One is proliferative, where new and fragile vessels grow in the retina, possibly as a result of poor oxygen supply from damaged initial blood vessels in the retina. The other, and most common, type of vision-threatening diabetic retinopathy is diabetic macular oedema where excessive leakiness of vessels occurs in the part of the retina that is critical to high-quality vision acuity termed the macula (and more centrally the fovea) [34]. In Australia, while diabetes remains the commonest cause of blindness in people of working age, less than 1% of people per year will develop blindness due to diabetes [35].
Diabetic nephropathy
While in each year only about 2% of people with diabetes develop very end-stage kidney disease (stage 5 chronic kidney disease), because diabetes mellitus is so common, diabetic 361nephropathy is the single commonest cause of end-stage kidney disease in Australia [35]. The damage to the kidney in diabetes may cause a progressive protein leak termed albuminuria, and also in the course of time, loss of kidney-filtering function (loss of the glomerular filtration rate). It is the combination of progressive albuminuria/proteinuria and loss of glomerular filtration rate that most clearly characterises diabetic nephropathy. It is thought that up to 30% of people with diabetes, in the course of time, will develop some nephropathy [36, 37]. In the early stages, small amounts of albumin loss in the urine, known as microalbuminuria, occur. Subsequently, the albumin and protein loss in the urine becomes more marked, and renal filtration function is lost. People with diabetic nephropathy not only have an increased risk of end-stage kidney failure developing but also increased cardiovascular disease risk – heart attack and heart failure [37].
Diabetic neuropathy
The commonest form of nerve damage in diabetes is to those supplying the feet. The sensory nerves are damaged more severely than the motor nerves. Loss of sensation in the feet increases the risk of foot ulceration in people with diabetes, and in their lifetime about 20% of people with diabetes will develop a foot ulcer, with less than 10% of these cases leading to the need for amputation [38]. In some people with diabetes, the feet and leg nerves become irritated and a painful form of neuropathy develops. This can be disabling, especially if prolonged, and requires specific medication to control, if not fully relieve, it.
There is debate as to whether the microvessels supplying the nerves (termed the ‘vasa nervorum’), or the nerves themselves, are directly affected by elevated glucose, with evidence from animal and human studies that both may be involved [38].
In addition to peripheral neuropathy, there are other forms of nerve damage that can occur in diabetes. These forms of neuropathy include those involving the automatic nerves of the body known as autonomic neuropathy. Autonomic neuropathy can cause heart rhythm problems and a proneness to low blood pressure on standing, as well as stomach and bowel upset. Rarer forms of neuropathy, which often improve over some months, involve the cranial nerves or and the nerve plexus in the pelvis, known as ‘diabetic amyotrophy’ [38].
B. Macrovascular complications
Cardiovascular disease
The commonest cause of death in people with diabetes is heart disease, accounting for about half of the deaths [10]. The heart can be affected by disease of the coronary arteries, which is increased two- to six-fold in people with diabetes [39]. The presentation of heart disease may be through a classic history of chest pain known as angina, although in some people with diabetes the symptoms may be more subtle, such as new shortness of breath on less exertion.362
Cerebrovascular disease
Lack of blood supply causing stroke is twice as common in people with diabetes as in the general population [40]. Large strokes can lead to death or major disability. In contrast, recurrent mini-strokes (causing damage to small parts of the brain known as ‘lacunes’), can lead over the years to a form of vascular dementia, especially if blood pressure is elevated.
Peripheral artery disease
Peripheral artery disease is more common in people with diabetes. It can cause a number of health problems such as calf pain on walking, and delayed healing in people who develop foot ulcers, thus increasing the risk of amputation [41]. Smoking markedly increases the risk of peripheral artery disease in people with diabetes, and for this and many other reasons smoking avoidance and cessation is vital in diabetes care.
C. Other organ complications in diabetes
There are many other complications that are caused by diabetes or may be related to it. Diabetic foot disease occurs due to combinations of neuropathy, peripheral artery disease, ulceration and infection and foot deformity [42]. Cataract and glaucoma are increased in people with diabetes [34]. Erectile dysfunction (some degree of impotence) is common in men with diabetes due to nerve damage and blood vessel injury [43]. Limited joint mobility and skin thickening can occur in people with diabetes [44]. Diabetes is known to directly adversely affect heart muscle to cause ‘diabetic cardiomyopathy’ in at least one-third of people [45]. In type 2 diabetes, fatty liver known as non-alcoholic liver disease occurs in most, and diabetes accelerates its rate of deterioration to an inflammatory and scarring form known as non-alcoholic steatohepatitis and in some cases across decades, liver cirrhosis [46, 47]. People with type 1 diabetes have an increased risk of developing immune related conditions that may affect the thyroid gland to cause over- or under-activity, and also the small bowel condition known as coeliac disease, which is a where effects of gluten in the diet can cause a toxic effect on the bowel [8].
(iii) Psychosocial complications in diabetes
Diabetes places major psychological stress and self-care demands on the person with diabetes and the caring family members. People with diabetes have an increased rate of clinical depression and eating disorders, which can exacerbate adverse organ effects of diabetes and also adversely affect quality of life [8]. Suicide rates may be higher in young people with diabetes compared with the general population [6]. There is also a diabetes condition known as ‘diabetes distress’ that describes an underlying anxiety and psychological unwellness in people with diabetes [8]. Diabetes can adversely affect psychosocial development in children and adolescence [8]. The increased frequency of these conditions reflects that psychsocial support is commonly indicated in those with diabetes.363
(iv) Clinical trials data in diabetes complications: metabolic control matters
While elevated blood glucose is a marker for increased diabetes complications risk, it took long-term clinical trials to prove that diabetes complications can be prevented by tight blood glucose control. Landmark studies in people with type 1 [8, 14] and type 2 diabetes [10, 48, 49, 50, 51, 52] have shown that better long-term average blood glucose, measured by a blood marker termed HbA1c or glycated haemoglobin, leads to reduced diabetes complications onset and in those who already have diabetes complications, their worsening. In studies varying from three to 10 years in duration, the onset and progression of microvascular complications were clearly prevented. For the macrovascular complications, cardiovascular events were shown to be prevented in longer-term studies of about 17 years’ duration [53, 54]. In those longer-term studies, tight blood glucose control in the first six to 10 years led to sustained reduction in the subsequent ten years or so, indicating that the body has a ‘memory’ effect of elevated blood glucose. This ‘metabolic memory’ is intriguing. Some recent preclinical data suggest that glucose regulation of factors that control genes, so-called epigenetics, is involved in the memory effect [55].
A recent study in type 2 diabetes indicates that very tight blood glucose control long term in people with cardiovascular disease may lead to increased mortality [51]. The exact cause of death in the study was unclear, although severe hypoglycaemia episodes causing heart attacks or abnormal heart rhythms may at least partly explain the increased death rate. This study has led to the concept that tight blood glucose control and targets are indicated early in diabetes, but in people with a history of cardiovascular disease, especially if they require insulin therapy, the blood glucose targets should be more relaxed [10, 56].
(v) Blood pressure and cholesterol control also prevent diabetes complications
A number of important studies in type 1 and type 2 diabetes have shown that blood pressure control can prevent onset and worsening of microvascular complications, especially diabetic nephropathy and diabetic retinopathy, and all macrovascular complications, especially stroke. The class of medications known as angiotensin converting enzyme inhibitors and angiotensin receptor antagonists/blockers, appear to have special protective roles and are the preferred first-line agents in people with diabetes [57]. Other studies have shown that reduction in blood levels of bad (LDL) cholesterol, especially with medications known as ‘statins’, even in those without known cardiovascular disease, will reduce the risk of cardiovascular complications such as heart attack and stroke [58].
A study of people with type 2 diabetes in Denmark examined whether the combined simultaneous targeting of blood pressure, cholesterol and blood glucose would lead to improved outcomes [59]. It clearly demonstrated that targeting all three end-points over eight years or more, led to a more than 50% reduction in macro- and microvascular complications. These studies form the evidence for targeting blood glucose, blood pressure and cholesterol in people with diabetes.364
(vi) Screening for diabetes complications
In people with diabetes, there is a clear evidence showing that screening to detect certain diabetes complications is important [8, 10]. This is because, in those developing complications, some therapies can be intensified to help prevent worsening of the complication to its end stages. For example, in diabetic retinopathy, vision-threatening forms (proliferative retinopathy or macular oedema) can be treated with laser therapy, or intraocular corticosteroids or anti-growth factor therapy, to help prevent vision loss. In diabetic nephropathy, even tighter blood pressure control (to less than 125/75 mmHg, rather than less than 130/80 mmHg) can help prevent its progression, and in people with diabetic foot disease, intensive patient education combined with protective foot wear and early treatment of foot ulcers, can help to prevent amputation.
The screening for diabetic retinopathy requires a thorough examination of the retinae and measurement of visual acuity. In diabetic nephropathy, urine tests for albumin and in some cases protein, as well as blood tests for renal function (glomerular filtration rate) are required. For neuropathy and foot problems, feet need to be carefully examined for sensation, pulses and mechanical foot problems as well as ulcers.
In people with type 2 diabetes, microvascular screening for complications should begin from diagnosis [10], as a delay in recognition of diabetes and diabetes diagnosis of some years is common in type 2 diabetes, and effects of high blood glucose often occur for many years before diabetes diagnosis. In type 1 diabetes, screening should occur after about three years of diabetes. In each case, screening should usually occur annually thereafter [8].
In terms of macrovascular disease, complications screening is usually by history taking and physical examination. The role for extensive heart investigations, including exercise tests, is unclear and studies have shown that unless a person has symptoms of heart disease, tight blood pressure, blood glucose and cholesterol control, as well as avoidance of smoking and possibly blood thinning (anti-platelet) therapy are the cornerstone of care.
In people with type 1 diabetes, screening approximately annually for autoimmune thyroid disease and also coeliac disease, each by blood test, is indicated in children and adults [8].
(vii) Major challenges in diabetes complications care
Managing diabetes and its complications has a number of inherent challenges. One dynamic tension is that long-term blood glucose control is important to prevent long-term organ complications of diabetes, yet tight blood glucose control can increase the risk of severe hypoglycaemia, especially in cases where insulin therapy is required, such as in all people with type 1 diabetes and in those with type 2 diabetes requiring insulin therapy. A second tension is that diabetes place major demands on a person’s lifestyle in terms of healthy diet and exercise and, simultaneously, people with diabetes usually need to take multiple preventive medications to manage blood glucose, blood pressure and cholesterol. The person with diabetes needs to self-monitor blood glucose multiple times daily using a finger prick device and people with type 1 diabetes need to inject insulin four to five times daily. In people with type 1 diabetes this equates to more than 1000 blood glucose self-tests 365and more than 1000 insulin injections yearly. Diabetes is unrelenting and does not provide any ‘holidays’.
Considering the demands that diabetes places on the person with diabetes and their families, the health professional support and healthcare delivery services required are extensive and high level. Services for people with diabetes need to be comprehensive yet individualised in their targets and emphasis [8, 10, 56, 60]. Multidisciplinary healthcare teams are required, involving a skilled doctor, diabetes nurse educator or practice nurse, and dietitian. In type 1 diabetes and complex type 2 diabetes, to support the general practitioner, an endocrinologist is indicated to help lead chronic care. Other members of the diabetes healthcare team will often include the podiatrist, exercise physiologist, psychologist or psychiatrist, orthotist, foot and vascular surgeon, renal physician, ophthalmologist, cardiologist and microbiologist. Care of the person with diabetes thus requires highly coordinated care in order to optimise targets in therapy and to ensure that regular diabetes complications screening is undertaken [8, 10, 56, 60].
(viii) Transplantation and diabetes complications
Kidney transplant is often an option in a person with diabetes who has end-stage renal failure requiring dialysis. Outcomes are good if cardiovascular disease and heart failure is not severe in a patient. Kidney transplant can be combined with pancreas transplant in some cases, resulting in reduced need for insulin therapy and a reduction in severe hypoglycaemia. There is also evidence that some forms of neuropathy and kidney disease can improve after pancreas transplant [61].
(ix) Reversibility of diabetes complications
While most diabetes complications are progressive, some are transient. The microvascular complications of diabetes may in some cases regress, especially with intensive therapy. For example, diabetic nephropathy in its early micro-albuminuria stages may regress to normo-albuminuria with treatment of blood pressure [62], and painful diabetic neuropathy symptoms may resolve with time and improved blood glucose control, as may early diabetic retinopathy. Even some changes of fibrosis in tissues such as the kidney may become less marked with tight control of blood pressure and blood glucose in diabetic nephropathy [61]. Most studies in diabetes complications to date have examined prevention of progression rather than regression of complications.
(x) Diabetes and obstetric care
It is beyond the scope of this chapter to address obstetric aspects of diabetes in detail. It should be noted however that in women with type 1 or type 2 diabetes, tight control of blood glucose before conception and across a pregnancy can lead to marked reduction in birth defects, stillbirth and large-at-birth babies [29, 30]. Outcomes are also improved for the baby in a mother with gestational diabetes, in terms of reduced complications at birth and possibly, less stillbirth [31]. The data from series of high quality studies, including Australian clinical trials [31], provides reassuring data that much can be achieved to 366prevent diabetes complications in pregnancies complicated by diabetes, whether it be type 1, type 2 or gestational diabetes in the mother.
(xi) Outcomes are improving in diabetes complications
Data from multiple databases in Australia and other developed countries have indicated that the rate of death in people with diabetes has reduced over the decades in both type 1 and type 2 diabetes [63, 64]. Factors that have likely contributed to this beneficial outcome include the increasing evidence to support more intensive control of cholesterol (with statins), blood pressure (with angiotensin converting enzyme inhibitors or angiotensin receptor blocker therapy) and blood glucose in people with diabetes, and possibly more complete screening for diabetes complications. Reductions in smoking rates have also likely helped. However, people with diabetes continue to have a reduced life expectancy on average compared with an age-matched general population and the earlier diabetes onset occurs, the greater the reduction in average life expectancy due to diabetes-related complications [63, 10]. This, combined with the end-stage complications that occur in diabetes and the morbidity complications caused and related healthcare costs [8, 10], demand no complacency in diabetes complications detection and its management.
(xii) Prospects in diabetes complications and related care
It is envisaged that healthcare services delivery will progressively be enhanced to ensure that most if not all people with diabetes have complications screening as clinically indicated and that they have their major risk factors of blood pressure, cholesterol and blood glucose tightly and safely controlled.
It may also be the case that an increasing focus on regression of diabetes complications will indicate whether actual regression of changes should be a target in treatment rather than just the prevention of worsening of complications. For example, regression of albuminuria may be shown in the course of time to predict fewer kidney and cardiovascular events than stabilisation of albuminuria levels alone. It may well be that our treatment of blood pressure improves with increasing and new combination of agents, as will our control of both good and bad cholesterol through combined therapies. In type 1 and type 2 diabetes improved knowledge in healthcare delivery and advances in technology, such as more sophisticated insulin pumps and blood glucose monitoring, will likely further help to improve long-term blood glucose diabetes and thus prevent organ complications and minimise severe hypoglycaemia.
Considering the adverse effects of high blood glucose on cells and cellular pathways to cause tissue injury (Figure 1), it is envisaged that therapies targeted at, for example, growth factors may increasingly help to prevent, stabilise and possibly reverse diabetes complications. Such is already being realised in diabetic retinopathy where anti-vascular endothelial growth factor therapy is becoming a reality for vision-threatening retinopathy to complement the traditional treatment of laser photocoagulation therapy [34]. As described in other chapters, the agent fenofibrate is showing remarkable effects in preventing microvascular complications of diabetes [65].367
Lastly, as interventions for diabetic cardiomyopathy in prevention of heart failure and diabetes and liver disease develop, it is envisaged that screening for these two conditions will improve. Worldwide, despite the epidemic of obesity and diabetes, increased access to insulin and blood glucose monitoring through programs such as Insulin for Life [66], and to skilled teams of healthcare professionals, through the support of the International Diabetes Federation and United Nations spearheaded campaigns, are expected to improve global outcomes in diabetes.
References
1. World Health Organization (2006). Definition and diagnosis of diabetes mellitus and intermediate hyoerglycemia report of a World Health Organization/IDF consultation. Geneva: World Health Organization Press.
2. Balasubramanyam A, Nalini R, Hampe CS & Maldonado M (2008). Syndromes of ketosis-prone diabetes mellitus. Endocrine Reviews, 29(3): 292–302.
3. Banting FG & Best CH (1987 [1922]). The internal secretion of the pancreas. The Journal of Laboratory and Clinical Medicine: Nutrition Classics, 45(2): 55–57.
4. Deckert T, Poulsen JE & Larsen M. (1978). Prognosis of diabetics with diabetes onset before the age of thirty-one. Diabetologia, 14: 363–77.
5. Foster DW & McGarry JD (1983). The metabolic derangements and treatment of diabetic ketoacidosis. New England Journal of Medicine, 309(3): 159–69.
6. Tu E, Twigg SM, Duflou C & Semsarian C (2008). Causes of death in young Australians with type 1 diabetes: a review of coronial post-mortem cases. Medical Journal of Australia,188(12): 699–702.
7. Wolfsdorf J, Craig ME, Daneman D, Dunger D, Edge J, Lee W, Rosenbloom A, Sperling M & Hanas R (2009). Diabetic ketoacidosis in children and adolescents with diabetes. Pediatric Diabetes, 10(Suppl. 12): 118–33.
8. Craig ME, Twigg SM, Donaghue KC, Cheung NW, Cameron FJ, Conn J, Jenkins AJ, Silink M, for the Australian Type 1 Diabetes Guidelines Expert Advisory Group (in press accepted 16 August, 2011). National evidence-based clinical care guidelines for type 1 diabetes in children, adolescents and adults. Canberra: Australian Government Department of Health and Ageing.
9. Kitabchi AE & Nyenwe EA (2006). Hyperglycemic crises in diabetes mellitus: diabetic ketoacidosis and hyperglycemic hyperosmolar state. Endocrinology and Metabolism Clinics of North America, 35(4): 725–51.
10. Colagiuri S, Dickinson S, Girgis S & Colagiuri R (2009). National evidence based guideline for blood glucose control in type 2 diabetes. Canberra: Diabetes Australia and the NHMRC.
11. Cryer PE, Axelrod L, Grossman AB, Heller SR, Montori VM, Seaquist ER, Service FJ & The Endocrine Society (2009). Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. Journal of Clinical Endocrinology & Metabolism, 94(3): 709–28.368
12. Anderbro T, Amsberg S, Adamson U, Bolinder J, Lins P, Wredling R, Moberg E, Lisspers J & Johansson UB (2010). Fear of hypoglycaemia in adults with type 1 diabetes. Diabetic Medicine, 27(10): 1151–58.
13. Pearson T (2008). Glucagon as a treatment of severe hypoglycemia: safe and efficacious but underutilized. The Diabetes Educator, 34(1): 128–34.
14. DCCT Research Group (Diabetes Control and Complications Trial Research Group) (1991). Epidemiology of severe hypoglycemia in the diabetes control and complications trial. American Journal of Medicine, 90(4): 450–59.
15. UKPDS Study Group (1998). Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes. (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. The Lancet, 352(9131): 837–53.
16. Australian Bureau of Statistics (2009). Causes of death in Australia [Online]. Available: abs.gov.au/AUSSTATS/abs@.nsf/mf/3303.0/ [Accessed 14 December 2011].
17. Bierhaus A & Nawroth PP (2009). Multiple levels of regulation determine the role of the receptor for AGE (RAGE) as common soil in inflammation, immune responses and diabetes mellitus and its complications. Diabetologia, 52(11): 2251–63.
18. Twigg SM & Cooper ME (2004). The time has come to target connective tissue growth factor in diabetic complications. Diabetologia, 47(6): 965–68.
19. Twigg SM (2010). Mastering a mediator: blockade of CCN-2 shows early promise in human diabetic kidney disease. Cell Communication and Signaling, 4(4): 189–96.
20. Brownlee M (2001). Biochemistry and molecular cell biology of diabetic complications. Nature, 414(6865): 813–20.
21. Jeong IK & King GL (2011). New perspectives on diabetic vascular complications: the loss of endogenous protective factors induced by hyperglycemia. Diabetes & Metabolism Journal, 35(1): 8–11.
22. Yu Liu D, Min T, Bolton V, Nubé SM, Twigg, DK Yue & SV McLennan (2009). Increased matrix metalloproteinase-9 predicts poor wound healing in diabetic foot ulcers. Diabetes Care, 32(1): 117–19.
23. McLennan SV, Wang XY, Moreno V, Yue DK & Twigg SM (2004). Connective tissue growth factor mediates high glucose effects on matrix degradation through tissue inhibitor of matrix metalloproteinase type 1: implications for diabetic nephropathy. Endocrinology, 145(12): 5646–55.
24. Min D, Lyons JG, Bonner J, Twigg SM, Yue DK & McLennan SV (2009). Mesangial cell-derived factors alter monocyte activation and function through inflammatory pathways: possible pathogenic role in diabetic nephropathy. American Journal of Physiology – Renal Physiology, 297(5): F1229–37.
25. Freedman BI, Bostrom M, Daeihagh P & Bowden DW (2007). Genetic factors in diabetic nephropathy. Clinical Journal of the American Society of Nephrology, 2: 1306–16.
26. Fitzgerald AP & Jarrett RJ (1991). Are conventional risk factors for mortality relevant in type 2 diabetes? Diabetic Medicine, 8(5): 475–80.
27. Castellino P, Tuttle KR & DeFronzo RA (1994). Diabetic nephropathy. Current Therapy in Endocrinology and Metabolism, 5: 426–36.369
28. McGill M, Molyneaux L, Twigg SM & Yue DK (2008). The metabolic syndrome in type 1 diabetes: does it exist and does it matter? Journal of Diabetes and its Complications, 22(1): 18–23.
29. Ray JG, O’Brien TE & Chan WS (2001). Preconception care and the risk of congenital anomalies in the offspring of women with diabetes mellitus: a meta-analysis. Quarterly Journal of Medicine, 94(8): 435–44.
30. Tieu J, Middleton P & Crowther CA (2010). Preconception care for diabetic women for improving maternal and fetal health. Cochrane Database of Systematic Reviews, 12: CD007776.
31. Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS & Robinson JS (2005). Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) Trial Group. The New England Journal of Medicine, 352(24): 2477–86.
32. Jacqueminet S & Jannot-Lamotte MF (2010). Therapeutic management of gestational diabetes. Diabetes & Metabolism, 36(6 Pt 2): 658–71.
33. Thomson SE, McLennan SV, Kirwan PD, Heffernan SJ, Hennessy A, Yue DK & Twigg SM (2008). Renal CTGF correlates with glomerular basement membrane thickness and prospective albuminuria in a non-human primate model of diabetes: possible predictive marker for incipient diabetic nephropathy. Journal of Diabetes and its Complications, 22(4): 284–94.
34. Australian Government Department of Health and Ageing (2008). Guidelines for the management of diabetic retinopathy. Canberra: Australian Government Department of Health and Ageing.
35. Australian Government Department of Health and Ageing (2009). Australian national diabetes information audit & benchmarking. Canberra: Australian Government Department of Health and Ageing.
36. Nathan DM, Zinman B, Cleary PA, Backlund JY, Genuth S, Miller R & Orchard TJ (2009). Modern-day clinical course of type 1 diabetes mellitus after 30 years’ duration: the diabetes control and complications trial/epidemiology of diabetes interventions and complications and Pittsburgh epidemiology of diabetes complications experience (1983–2005). Archives of Internal Medicine, 169: 1307–16.
37. Lehmann R & Schleicher ED (2000). Molecular mechanism of diabetic nephropathy. Clinica Chimica Acta, 297: 135–44.
38. Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A, Bernardi L, Valensi P & Toronto Diabetic Neuropathy Expert Group (2010). Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care, 33(10): 2285–93.
39. Ford ES (2011). Trends in the risk for coronary heart disease among adults with diagnosed diabetes in the US: findings from the National Health and Nutrition Examination Survey (1999–2008). Diabetes Care, 34(6): 1337–43.
40. Kothari V, Stevens RJ, Adler AI, Stratton IM, Manley SE, Neil HA & Holman RR (2002). UKPDS 60: risk of stroke in type 2 diabetes estimated by the UK Prospective Diabetes Study risk engine. Stroke, 33(7): 1776–81.370
41. Adler AI, Stevens RJ, Neil A, Stratton IM, Boulton AJ & Holman RR (2002). UKPDS 59: hyperglycemia and other potentially modifiable risk factors for peripheral vascular disease in type 2 diabetes. Diabetes Care, 25(5): 894–99.
42. Australian Government Department of Health and Ageing (2011). National evidence-based guideline: prevention, identification and management of foot complications in diabetes. Canberra: Australian Government Department of Health and Ageing.
43. Hermans MP, Ahn SA & Rousseau MF (2009). Erectile dysfunction, microangiopathy and UKPDS risk in type 2 diabetes. Diabetes & Metabolism, 35(6): 484–89.
44. Kordonouri O, Maguire AM, Knip M, Schober E, Lorini R, Holl RW & Donaghue KC (2009). Other complications and associated conditions with diabetes in children and adolescents. Pediatric Diabetes, 10(Suppl. 12): 204–10.
45. Brooks BA, Franjic B, Ban CR, Swaraj K, Yue DK, Celermajer DS & Twigg SM. (2008). Diastolic dysfunction and abnormalities of the microcirculation in type 2 diabetes. Diabetes, Obesity and Metabolism, 10(9): 739–46
46. Lo L, McLennan SV, Williams PF, Bonner J, Chowdhury S, McCaughan GW, Gorrell MD, Yue DK & Twigg SM (2011). Diabetes is a progression factor for hepatic fibrosis in a high fat fed mouse obesity model of non-alcoholic steatohepatitis. Journal of Hepatology, 55(2): 435–44.
47. Clark JM (2006). The epidemiology of nonalcoholic fatty liver disease in adults. Journal of Clinical Gastroenterology, 40: S5–S10.
48. UKPDS Study Group (1998). Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes. (UKPDS 34). The Lancet, 352(9131): 854–65.
49. UKPDS Study Group (1998). Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes. (UKPDS 33). The Lancet, 352(9131): 837–53.
50. ADVANCE Collaborative Group (2008). Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. New England Journal of Medicine, 358(24): 2560–72.
51. ACCORD Study Group (2008). Effects of intensive glucose lowering in type 2 diabetes. New England Journal of Medicine, 358(24): 2545–59.
52. Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG & Huang GD (2009). Glucose control and vascular complications in veterans with type 2 diabetes. New England Journal of Medicine, 360(2): 129–39.
53. Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B & DCCT/EDIC Study Research Group (2005). Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes, New England Journal of Medicine, 353(25): 2643–53.
54. Holman RR, Paul SK, Bethel MA, Matthews DR & Neil HA (2008). 10-year follow-up of intensive glucose control in type 2 diabetes. New England Journal of Medicine, 359(15): 1577–89.371
55. Tonna S, El-Osta A, Cooper ME & Tikellis C (2010). Metabolic memory and diabetic nephropathy: potential role for epigenetic mechanisms. Nature Reviews Nephrology, 6: 332–41.
56. Cheung NW, Conn JJ, d’Emden MC, Gunton JE, Jenkins AJ, Ross GP, Sinha AK, Andrikopoulos S, Colagiuri S & Twigg SM (2009). Position statement of the Australian Diabetes Society: individualisation of glycated haemoglobin targets for adults with diabetes mellitus. Medical Journal of Australia, 191(6): 339–44.
57. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr & Roccella EJ (2003). The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. Journal of the American Medical Association, 289(19): 2560–72.
58. Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason MJ, Mackness MI, Charlton-Menys V, Fuller JH & CARDS investigators (2004). Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. The Lancet, 364(9435): 685–96.
59. Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH & Pedersen O (2003). Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. New England Journal of Medicine, 348(5): 383–93.
60. Colagiuri R, Girgis S, Eigenmann C, Gomez M & Griffiths R (2009). National evidence based guideline for patient education in type 2 diabetes. Canberra: Diabetes Australia and the NHMRC.
61. Han DJ & Sutherland DE (2010). Pancreas transplantation. Gut Liver, 4(4): 450–65.
62. Ismail-Beigi F, Craven T, Banerji MA, Basile J, Calles J, Cohen RM, Cuddihy R, Cushman WC, Genuth S, Grimm RH Jr, Hamilton BP, Hoogwerf B, Karl D, Katz L, Krikorian A, O’Connor P, Pop-Busui R, Schubart U, Simmons D, Taylor H, Thomas A, Weiss D, Hramiak I & ACCORD trial group (2010). Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. The Lancet, 376(9739): 419–30.
63. Secrest AM, Becker DJ, Kelsey SF, LaPorte RE & Orchard TJ (2010). All-cause mortality trends in a large population-based cohort with long-standing childhood-onset type 1 diabetes: the Allegheny County type 1 diabetes registry, Diabetes Care, 33(12): 2573–79.
64. Gu K, Cowie CC & Harris MI (1999). Diabetes and decline in heart disease mortality in US adults. Journal of the American Medical Association, 281(14): 1291–97.
65. Rajamani K, Colman PG, Li LP, Best JD, Voysey M, D’Emden MC, Laakso M, Baker JR, Keech AC & FIELD study investigators (2009). Effect of fenofibrate on amputation events in people with type 2 diabetes mellitus (FIELD study): a prespecified analysis of a randomised controlled trial. The Lancet, 373(9677): 1780–88.
66. Insulin for Life website: www.insulinforlife.org/