16
The role of physical activity in the prevention and treatment of diabetes
This chapter is concerned with the role of physical activity and exercise prescription as components in the prevention and treatment of diabetes. First, the evidence for the diabetes-specific health benefits of physical activity is summarised and the physiological mechanisms are pointed out. Studies of medical comorbidities of diabetes are discussed. Recommendations for physical activity and exercise in the prevention and control of type 2 diabetes are provided, and challenges in the implementation of such strategies are discussed.
The health benefits of regular moderate-intensity physical activity are well known. In particular, epidemiological evidence has shown that an active lifestyle is beneficial in the prevention and treatment of more than 20 health conditions including coronary heart disease, stroke, type 2 diabetes and some cancers [1, 2, 3].
Physical activity is defined as body movement produced by skeletal muscle that results in energy expenditure above resting level [2], and can be accumulated at any time, for example during work, housework, transportation or during leisure time. Exercise is a subset of ‘leisure time physical activity’ that is usually structured, planned, repetitive, and has the purpose of providing recreation, improving or maintaining physical fitness, or enhancing other components of health or wellbeing (see Figure 1). Physical fitness includes cardio-respiratory fitness, muscle strength, body composition and flexibility [4, 5]. Metabolic fitness is also increasingly recognised as an important component of fitness which is closely related to physical activity levels, as well as cardiovascular and musculoskeletal fitness [6].
For the general adult population the American College of Sports Medicine recommends to accumulate at least 30 minutes of at least moderate-intensity physical activity on five, preferably all, days of the week, or vigorous-intensity aerobic physical activity for at least 20 minutes on three days per week [1, 3, 7]. However, even though physical activity confers numerous health benefits, large parts of the adult population are not sufficiently active [8, 9, 10, 11, 12, 13].
276Type 2 diabetes is related to genetic, environmental and behavioural factors. Particularly, lack of physical activity and visceral obesity are considered to be major contributors to the global diabetes epidemic.
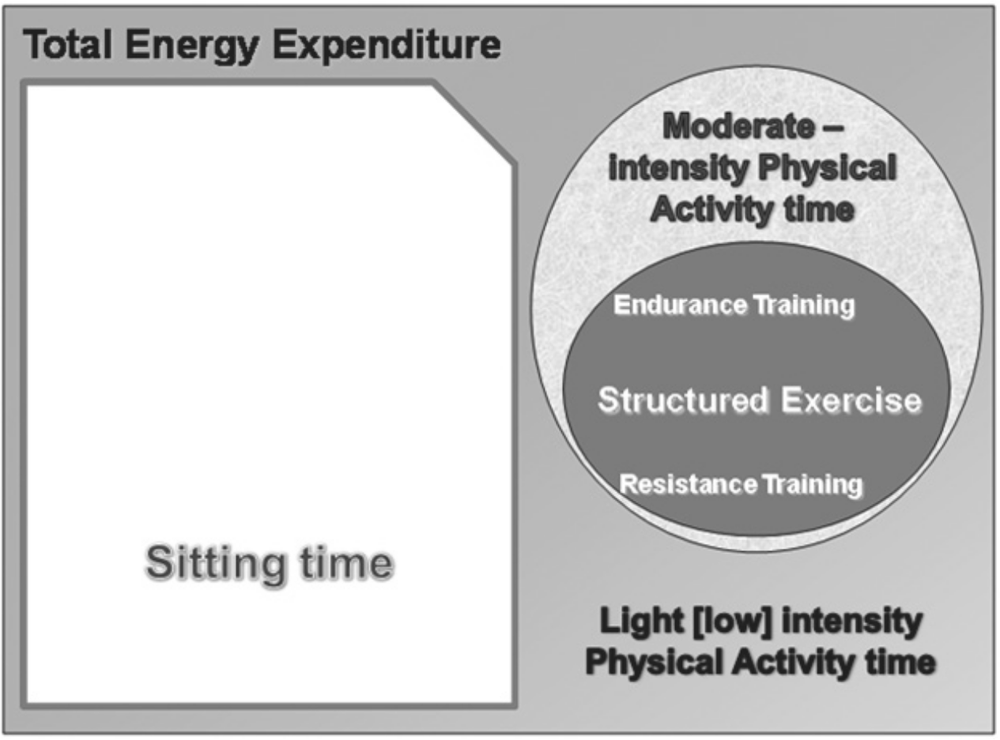
Figure 1. Energy expenditure in humans subdivided into sedentary behaviour, physical activity and the various forms of exercise.
This chapter is concerned with the role of physical activity and exercise prescription as components in the prevention and treatment of diabetes [14, 15]. First, the evidence for the diabetes-specific health benefits of physical activity will be summarised. Recommendations for physical activity and exercise in the prevention and control of type 2 diabetes will be provided, and challenges in the implementation of such strategies will be discussed.
Prevention of type 2 diabetes
Physical inactivity and overweight/obesity are both risk factors for the development of type 2 diabetes. Regular physical activity improves insulin sensitivity, and reduces glucose levels [16]. The role of obesity in the development of diabetes type 2 is likely to work through adipocytes releasing adipocytokines into the circulation, including leptin, adipsin, resistin and interleukin-6 (IL-6) [17], some of which are associated with insulin resistance [18, 19]. There are indications that obesity plays a larger role than physical activity in the development of diabetes [20, 21, 22]. However, physical activity has been shown to be beneficial in the prevention of type 2 diabetes independent of weight loss [16]. Besides the beneficial effects of physical activity on the development of type 2 diabetes, it can also be effective in prevention of the metabolic syndrome [16, 23], and during pregnancy in the prevention of gestational diabetes [24, 25], and has independent benefits in reducing cardiovascular risk [1]. The following sections summarise the evidence from observational and intervention 277studies on the role of physical activity in the primary and secondary prevention of type 2 diabetes.
Diabetes prevention evidence: observational studies
The evidence for primary prevention comes from observational epidemiology; these are usually from large population-based cohort studies that examined whether exposure to occupational, commuting and leisure-time physical activity was related to the subsequent risk of developing type 2 diabetes.
A meta-analysis synthesised the findings from ten prospective cohort studies from the US, the UK, Finland and Japan regarding the exposure to moderate-intensity physical activity and the risk of developing type 2 diabetes [26]. These studies included 301 221 participants and 9367 incident cases of diabetes. The pooled relative risk for diabetes in those who regularly engaged in moderate physical activity was 0.69 (95% CI 0.58–0.83) compared with inactive participants. Similarly, the relative risk of those who walked regularly was 0.70 (0.58–0.84) compared with almost no walking. The associations remained significant when adjusted for BMI [26].
Likewise, a recent report summarised the results from 25 prospective cohort studies and found a similar inverse relationship between physical activity and diabetes incidence. This relationship applied to men and women, different age groups and ethnicities. The reduction in relative risk for type 2 diabetes ranged from 15%–60% for those that were physically active compared with their more inactive peers [16]. It also appears that several domains of physical activity (occupational, commuting and leisure-time physical activity) are all inversely related to the risk of developing type 2 diabetes. Based on these studies, at least 30 minutes per day (210 minutes per week) of at least moderate-intensity physical activity is sufficient to achieve a significant reduction in diabetes incidence [16].
A new area of research has developed around total ‘sitting’ time. These studies have indicated that prolonged sitting time, including measures of television watching, might be associated with the risk of developing type 2 diabetes, independent of leisure-time physical activity [27, 28, 29]. Furthermore, breaks in sitting time have been associated with improvements in waist circumference, triglycerides, and two-hour plasma glucose levels [30]. Two large cohort studies have observed that each two-hour increment per day in watching TV, as a proxy measure of sitting time, was associated with a 14%–20% increase in diabetes incidence, even after adjustment for physical activity participation [31, 32].
Diabetes prevention: the evidence from intervention studies
The strongest evidence for the benefits of physical activity and exercise in the prevention of type 2 diabetes comes from community-based lifestyle intervention trials. To date, at least six randomised trials have examined whether lifestyle interventions, including physical activity, reduce the risk of developing type 2 diabetes among adults with impaired glucose tolerance. These trials are:278
- Diabetes Prevention Program (DPP), US
- Diabetes Prevention Study (DPS), Finland
- Da Qing IGT and Diabetes Study (DQS), China
- Diabetes Prevention Program (IDPP), India
- Diabetes Prevention Program (JDPP), Japan
- Västerbotten Intervention Program (VIP), Sweden.
All these trials demonstrated that structured lifestyle modification programs reduced the incidence of type 2 diabetes compared to controls or usual care. For these programs, exercise was usually an independent predictor of improved metabolic control or reduced diabetes incidence, and varied modes of exercise were usually included (see Table 1).
In the Da Qing study in China, 577 middle-aged men and women with impaired glucose tolerance were randomised to a diet only, exercise only, diet and exercise, or control group. The unsupervised exercise prescription ranged from 140 minutes per week for those over 50 years to 280 minutes per week for younger participants. After six years, the diet, exercise, and combined diet and exercise interventions achieved 31%, 46%, and 42% reductions in the risk of developing diabetes compared with the control group [33]. At 20-year follow-up, a sustained preventive benefit was still observed, with the likelihood of diabetes 43% lower among those allocated to the lifestyle interventions [34].
In the US Diabetes Prevention Program (DPP) 3324 overweight and obese participants with impaired glucose tolerance were randomised into three groups (placebo, metformin therapy, and lifestyle). The lifestyle modification intervention aimed to increase physical activity to at least 150 minutes per week (primarily unsupervised brisk walking, but with availability of two sessions of supervised exercise per week) and reduce weight by at least 7%. After a follow-up of three years the lifestyle intervention showed a 58% reduction in the risk of developing diabetes compared to the placebo group, while the metformin group had a risk reduction of only 31% [35]. At ten-year follow-up, compared to controls, the lifestyle intervention maintained a 34% lowered risk of diabetes, and the metformin group maintained an 18% reduction [36].
The Finnish Diabetes Prevention Study (n = 522) showed similar results to the US DPP. This lifestyle intervention comprised 210 minutes per week of exercise (including three sessions per week of supervised aerobic and resistance/power training) and diet-induced weight loss of 5%–7%, resulting in a four-year diabetes risk reduction of 58% (p <.001). In this study, those who failed to meet the weight-loss goal, but accumulated four hours of weekly moderate exercise, had a significantly lower risk of diabetes than the control group [37]. Three years after the program, intervention group participants maintained many lifestyle changes and their risk for diabetes was still 36% lower than among controls [38].279
Table 1. Lifestyle change goals in the intervention groups in diabetes prevention programs. Adapted from Baker M et al. 2011.
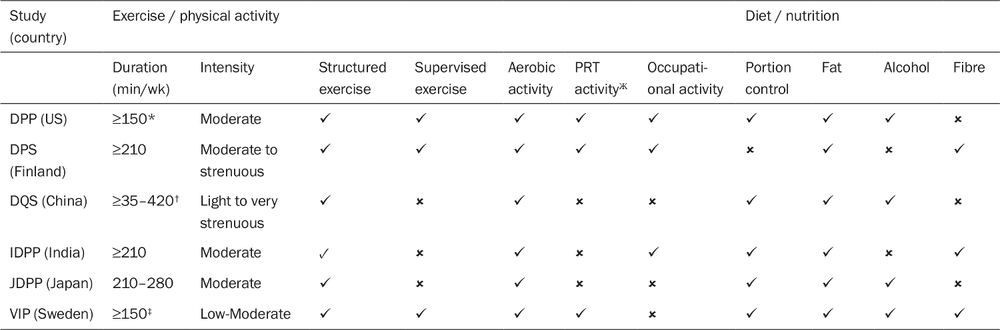
* Energy expenditure ≥700 kcal/week was the primary objective, and the target volume (min/week) was allowed to be increased/decreased based on the intensity of activities performed.
† 35–210 min/week for persons ≥50 years; 70–420 min/week for persons <50 years.
‡ 2.5 hours per day prescribed as supervised exercise in the first month. PRT = Progressive resistance training; DPP = Diabetes Prevention Program (US); DPS = Diabetes Prevention Study (Finland); DQS = Da Qing IGT and Diabetes Study (China); IDPP = Diabetes Prevention Program (India); JDPP = Diabetes Prevention Program (Japan); VIP = Västerbotten Intervention Program (Sweden).
Ж PRT: progressive resistance training.
280The Indian Diabetes Prevention Study (n = 531) comprised a physical activity target of unsupervised brisk walking for at least 30 minutes each day (210 min/week). After three years the relative risk reduction for diabetes was 28.5% for the lifestyle intervention, 26.4% for metformin, and 28.2% for the combination of both interventions compared to the control group [39].
A non-randomised lifestyle intervention among middle-aged males in Sweden included a substantial physical activity component. Over six years of follow-up, the lifestyle intervention achieved a 63% risk reduction for the development of diabetes compared with the control group [40]. A more recent Swedish study randomised 194 middle-aged adults with impaired glucose tolerance to lifestyle intervention or usual care, and observed metabolic indicators and insulin resistance improvements at 12 months, with some improvements maintained at five years (VIP program) [41].
The Japanese Diabetes Prevention Program (JDPP) followed 458 men for four years. Twenty percent of participants were randomised to an intensive intervention group of diet and physical activity advice, and the others were assigned to the standard intervention (control) group. The JDPP prescribed 30 to 40 minutes per day (ie 210 to 280 minutes per week) of unsupervised moderate intensity physical activity [42]. This prescription included sport and active commuting, and reduced diabetes incidence by 68% at four-year follow-up.
Summary of diabetes prevention evidence
The net results of these diverse trials in people at risk of diabetes show a clear secondary prevention benefit of lifestyle interventions [43]. There appear to be independent protective benefits of both weight loss and physical activity. A recent Cochrane review summarised these results, and in a meta-analysis of randomised controlled diabetes prevention trials, showed that exercise and diet interventions reduced the risk of type 2 diabetes by 37% [44]. Although most lifestyle trials did not distinguish benefits attributed to weight loss or to physical activity [45], several studies did observe effects of activity independent of weight loss, suggesting these risk factors partly operate independently of each other [33, 39].
Limitations of these trials include self-report of physical activity participation. However, in epidemiological studies where measurement error in exposure occurs, the observed relative risk is likely to underestimate the true relative risk, so these ‘physical activity self report’ estimates are likely to underestimate the preventive relationship between activity and diabetes incidence. Objective measurement would be better [46], and in epidemiological studies, fitness measures show a strong protective relationship to diabetes incidence [47, 48, 49].
Recommendations for diabetes prevention
For at-risk individuals, a minimum dose of at least 150 minutes per week, and possibly substantially more, of ‘at least moderate-intensity physical activity’ is recommended to prevent diabetes. This is in addition to recommendations for a healthy diet and weight loss. 281Physical activity advice should form part of every clinical counselling session with individuals at risk of diabetes, and a clear plan should be developed. People can accumulate physical activity in different settings: through active commuting, and in their leisure time through structured or unstructured exercise programs. Higher levels of physical activity, up to an hour a day of moderate-intensity activity, yield even greater reductions in the incidence of type 2 diabetes [1, 43, 50]. Resistance training regimens, which are important in diabetes management, should also be considered, especially for older adults [3], and three of the RCTs of diabetes prevention (DPP, DPS, VIP) have specifically included this exercise modality. It also appears that long uninterrupted periods of sitting should be avoided.
As previously mentioned, obesity is an independent risk factor for diabetes. However, while a minimum of 30 minutes of physical activity per day (150 to 210 minutes per week) appears to be sufficient for a significant risk reduction in type 2 diabetes, this may not be sufficient for weight maintenance or weight loss [3, 51]. At least 60 to 90 minutes of moderate-intensity physical activity daily is needed for weight maintenance in previously overweight or obese people [51, 52, 53]. Therefore, engaging in more than 30 minutes of moderate-intensity physical activity per day would not only reduce the risk of developing diabetes directly, but for those who are overweight or obese also by influencing body weight and improving fat distribution.
1.5 Challenges in implementation
The epidemiological evidence relates to both the primary and secondary prevention of diabetes. The primary prevention of diabetes involves a whole-population approach, which requires actions in both clinical and community settings. This means that all clinical encounters need to recommend physical activity/exercise, in the same way that tobacco cessation is almost universally recommended. Unfortunately, many primary care physicians remain reluctant to adopt this area of preventive counselling [54]. Advice regarding moderate intensity physical activity may have better compliance rates than vigorous-intensity prescriptions [55], so that the initial goal is to ensure all sedentary and low active individuals reach at least the minimal threshold of 150 minutes of activity each week, preferably across five or more days. In addition, clinicians need to become advocates for physical activity programs and facilities across the community [56], in the same way that they rapidly adopted and disseminated an anti-smoking stance in the 1980s.
The secondary prevention goal requires identification of those at risk for diabetes, which is itself a challenge. For these individuals, more structured and evidence-based lifestyle advice is important. Patient advice should highlight the importance of physical activity in diabetes prevention, and include referral to appropriate structured exercise programs; in addition, some increases in incidental lifestyle activities are recommended to increase total energy expenditure, including active transport, increasing active chores and being active in one’s local environment.282
Management of type 2 diabetes
Apart from its role in the prevention of type 2 diabetes, physical activity and exercise have important roles in the treatment and management of diabetes [57, 58, 59, 60]. This role remains largely unincorporated into mainstream clinical management of type 2 diabetes [61]. Given that exercise has an effect as potent as most oral hypoglycaemic agents [62], this is a critical gap in clinical care.
In this section, the health benefits of physical activity and exercise for individuals with type 2 diabetes will be discussed. Firstly, the evidence for the health benefits from various physical activity and exercise interventions for individuals with type 2 diabetes will be described. Then, physical activity and exercise recommendations for the care of diabetes will be outlined. Lastly, challenges in the implementation of strategies to increase levels of physical activity participation among those with diabetes will be discussed.
Diabetes management evidence: aerobic training
The majority of studies on the role of physical activity in the management of type 2 diabetes have focused on the effects of aerobic training [63]. Aerobic exercise involves the usage of large muscles and relies on aerobic levels of energy expenditure (eg brisk walking, cycling, jogging, swimming). Aerobic exercise alone is associated with clinically significant reductions in the glycosylated haemoglobin level [64, 65]. The physiological adaptations responsible for improvements in glucose control through aerobic training include increased capillary density, glucose transport (GLUT4) proteins in muscle, protein kinase B content, and glycogen synthase activity, as well as a shift from low-oxidative type 2b muscle fibres to moderate-oxidative, more insulin-sensitive type 2a muscle fibres [63, 66], and decreased inflammatory cytokines which impair insulin signalling in skeletal muscle [67, 68].
Aerobic training improves fitness levels and insulin sensitivity, and also results in redistribution of visceral adiposity [69]. Even in the absence of weight loss, vigorous activity can improve insulin sensitivity [70], decrease insulin resistance and reduce arterial stiffness [71]. Diabetes markers improved by exercise include insulin resistance, but also HDL and lipid profiles, as well as glycosylated haemoglobin levels and systemic inflammation in some studies [72, 73].
A meta-analysis pooled the results of seven randomised controlled trials on the effects of structured aerobic exercise interventions on cardio-respiratory fitness in adults with type 2 diabetes (n = 266). Participants in the exercise groups increased their fitness (VO2max) by 11.8%, compared to 1% in control groups (p < 0.003). Interventions with higher intensities tended to yield larger improvements in fitness. Moreover, exercise intensity predicted weighted mean difference in glycosylated haemoglobin better than exercise volume [62]. A recent large-scale randomised trial from the US (n = 4376 overweight and obese individuals with type 2 diabetes) also found increases in cardio-respiratory fitness to be significantly higher in a combined physical activity and diet intervention than in a diabetes support and education group [74].283
Diabetes management evidence: resistance training
Traditionally, aerobic training has been used as the main exercise modality to manage type 2 diabetes [75]. However, in recent years resistance training has been gaining wide acceptance as an important strategy in the treatment of diabetes [63]. In resistance training muscles work against a resistive load or weight leading to hypertrophy and improved muscular strength. With increasing age adults have decreases in muscle mass, functional capacity, resting metabolic rate, and increases in adiposity, and insulin resistance. Resistance training is associated with improvements in bone mineral density, muscle strength and muscle hypertrophy and can thereby help in the prevention of osteoporosis and sarcopenia and in the maintenance of functional status [7, 76]. However, only a small number of wellcontrolled intervention studies have examined the benefits of resistance training for people with type 2 diabetes [63]. The most recent meta-analysis of resistance training by Strasser et al. [77] included 13 RCTs in which resistance training reduced glycosylated haemoglobin (HbA(1c)) by 0.48% (95% CI –0.76, –0.21; p = 0.0005), fat mass by 2.33 kg (95% CI –4.71, 0.04; p = 0.05) and systolic blood pressure by 6.19 mmHg (95% CI –11.38, –1.00; p = 0.02). It has been hypothesised that the improved glucose uptake is not only due to an increase in muscle mass that is associated with resistance training, but probably also due to qualitative changes in muscles that enhance insulin signalling and thereby sensitivity [78]. Furthermore, progressive resistance training (PRT) has been found to positively influence insulin resistance [79, 80]. Based on this evidence, resistance training should be recommended in the management of type 2 diabetes and metabolic disorders.
The strongest evidence for the health benefits of resistance training in type 2 diabetes comes from two trials that used multiple exercises at relatively high intensities and showed a decrease in glycosylated haemoglobin of 1.1%–1.2% in the intervention group compared to no significant change in the control group [81, 82]. However, even studies that used relatively low volumes and intensities of resistance training achieved positive effects on glucose control [79, 83, 84, 85, 86, 87]. This is particularly important for individuals who are totally sedentary and are not likely to participate in programs involving strenuous aerobic or resistance training [63]. This could apply to older adults, who may find it difficult to get to, or participate in, structured aerobic programs.
Other health benefits of resistance training in diabetes are an improved body composition, increases in total fat-free mass, reduced blood pressure [82], improved muscular strength [85], lipid profiles [84], bone mineral density and metabolic rate at rest, and a preferential mobilisation of visceral and subcutaneous adipose tissue in the abdominal region [63]. Resistance training can also help in reducing the required insulin-sensitising medication dose and thereby limit side effects [82, 88], and benefits depression [89] and cognitive impairment [90], both of which are more prevalent in those with diabetes.
While there is substantial evidence for the health benefits of resistance training for people with type 2 diabetes, a Canadian study showed that 88% of individuals with diabetes do not carry out resistance training activities [91]. This shows the unrealised potential of wide-scale interventions to promote resistance training among people with diabetes.284
Diabetes management evidence: aerobic and resistance training
Resistance and aerobic training have similar beneficial effects on insulin sensitivity and metabolic control in type 2 diabetes [58, 64, 92]. However, some studies compared combinations of both resistance and aerobic exercise versus only one of the two exercise modalities. A Canadian study (n = 28) compared the effects of a combined aerobic and resistance training program to aerobic training only in postmenopausal women with type 2 diabetes. There were no differences in weight loss, fitness, or blood lipids, but the combined group showed better glucose uptake and larger increases in muscle mass compared to the aerobic only group [87]. Similarly, an American study also reported on benefits of combining resistance and aerobic training on glycaemic control, fitness and lean tissue mass [65]. Another Canadian study showed that while both aerobic and resistance training alone were effective in reducing glycosylated haemoglobin, the combination of both exercise modalities was more effective [64]. However, in this study, the combined group had twice the volume of exercise, which may have been responsible for the added efficacy of this treatment, as noted by the authors. Furthermore, an Italian study (n = 120) that combined aerobic and resistance training showed more general improvements in cardiovascular risk factors than studies that used resistance training only as the intervention [93]. Finally, a meta-analysis found that the differences in the benefits of aerobic, resistance and combined training for people with diabetes were small. However, combined training generally showed advantages over aerobic or resistance training alone [94]. This leads to the optimal clinical recommendation of both modalities, but either may be more convenient or accessible for different patient groups.
Clinically, there are many patients with multiple comorbidities who cannot tolerate the dose of aerobic exercise that has proven effective in RCTs. Resistance training improves aerobic capacity, osteoarthritis pain and disability, depression, functional status, gait and balance impairments, bone density, and insomnia, thus addressing the spectrum of associated clinical disorders in older type 2 adults with diabetes [95], and providing a strong rationale for its utility in this condition. Notably, high intensity PRT is feasible even when robust aerobic exercise is impossible due to frailty, balance disorders, osteoarthritis or advanced peripheral vascular disease for example, making it particularly suitable for the typical older adult with type 2 diabetes. In addition, only PRT attenuates the loss of lean tissue (muscle and bone) accompanying weight loss diets typically prescribed for overweight adults with diabetes [96], thus minimising weight cycling related to lowered basal metabolic rate. By contrast, aerobic exercise is not anabolic, and does not increase muscle mass, precluding the associated metabolic and clinical benefits such a shift in body composition produces [13].
Diabetes management evidence: patho-physiological mechanisms
A Cochrane review and a meta-analysis, both synthesising the results of 14 randomised controlled trials of aerobic and resistance exercise programs, found that levels of glycosylated haemoglobin were reduced by around 0.6% in the exercise groups, while there were no differences between the groups in body mass index [97, 98]. Khaw et al. found that each 1% increase in glycosylated haemoglobin with levels between 5%–6.9% was associated with 285a 28% increase in mortality risk, independent of other risk factors, including age, blood pressure, serum cholesterol and body mass index [99]. It was suggested that much of the excess mortality risk of diabetes in men could be profiled by the biomarker of increased glycosylated haemoglobin.
The limited impact on the body mass index in exercising groups may be due to increases in muscle mass, that keep body mass relatively constant [97], particularly in trials of resistance training. It seems likely that muscle and adipose tissue distribution plays a crucial role in glucose metabolism, rather than body mass itself. It has been shown that an increase in lean body mass is inversely correlated with changes in glycosylated haemoglobin [83, 100], which might be due to increased storage of glucose in the skeletal muscle [76]. Muscle hypertrophy is not only associated with an increase in insulin sensitivity, but also with an increase in resting metabolic rate, exercise tolerance and functional mobility [65]. Furthermore, exercise-induced reductions in abdominal subcutaneous and visceral adipose tissue distributions are related to increased insulin sensitivity, without changes in overall body weight [69, 87]. Adipose tissue is associated with secretion of adipocytokines, which can negatively influence insulin resistance [101]. In conclusion, exercise-induced increases in fat-free mass and reductions in adipose tissue, as well as shifts in anabolic-catabolic hormonal profile are beneficial for glucose control and other aspects of metabolic health, regardless of changes in body weight. These relationships are demonstrated in Figure 2.
Summary of diabetes management evidence
Numerous trials have demonstrated the health benefits of physical activity and exercise in the treatment of type 2 diabetes. Physical activity is associated with improved insulin sensitivity and glucose control independent of weight loss [97]. Moreover, regular physical activity leads to a decrease in blood lipids and blood pressure, and has independent protective benefits on coronary heart disease risk, which is elevated among those with diabetes. The benefits achieved by aerobic and resistance training seem apparent shortly after starting a structured exercise program. Activity needs to be regular; the frequency of exercise sessions is optimally at least three or more times per week to maintain metabolic benefits. This is due to the known acute bout effect of exercise on insulin resistance, which wanes between 24 and 96 hours after exercise, and is responsible for a portion of the long-term training benefits [102]. In general, both higher volumes and higher intensities of exercise result in greater metabolic improvements [43].
There are a few limitations in studies on physical activity in diabetes management, including trials in small selected samples. Similar to studies about the prevention of diabetes, some trials used interventions of combined physical activity, nutrition and medication [74, 103]. In order to isolate the independent effect of physical activity in diabetes management interventions, one needs to compare exercise alone versus other therapeutic modalities [97]. Current trials are underway examining the long-term efficacy of exercise and dietary 286interventions in the treatment of type 2 diabetes, such as the Look AHEAD study [104, 105, 106, 107].
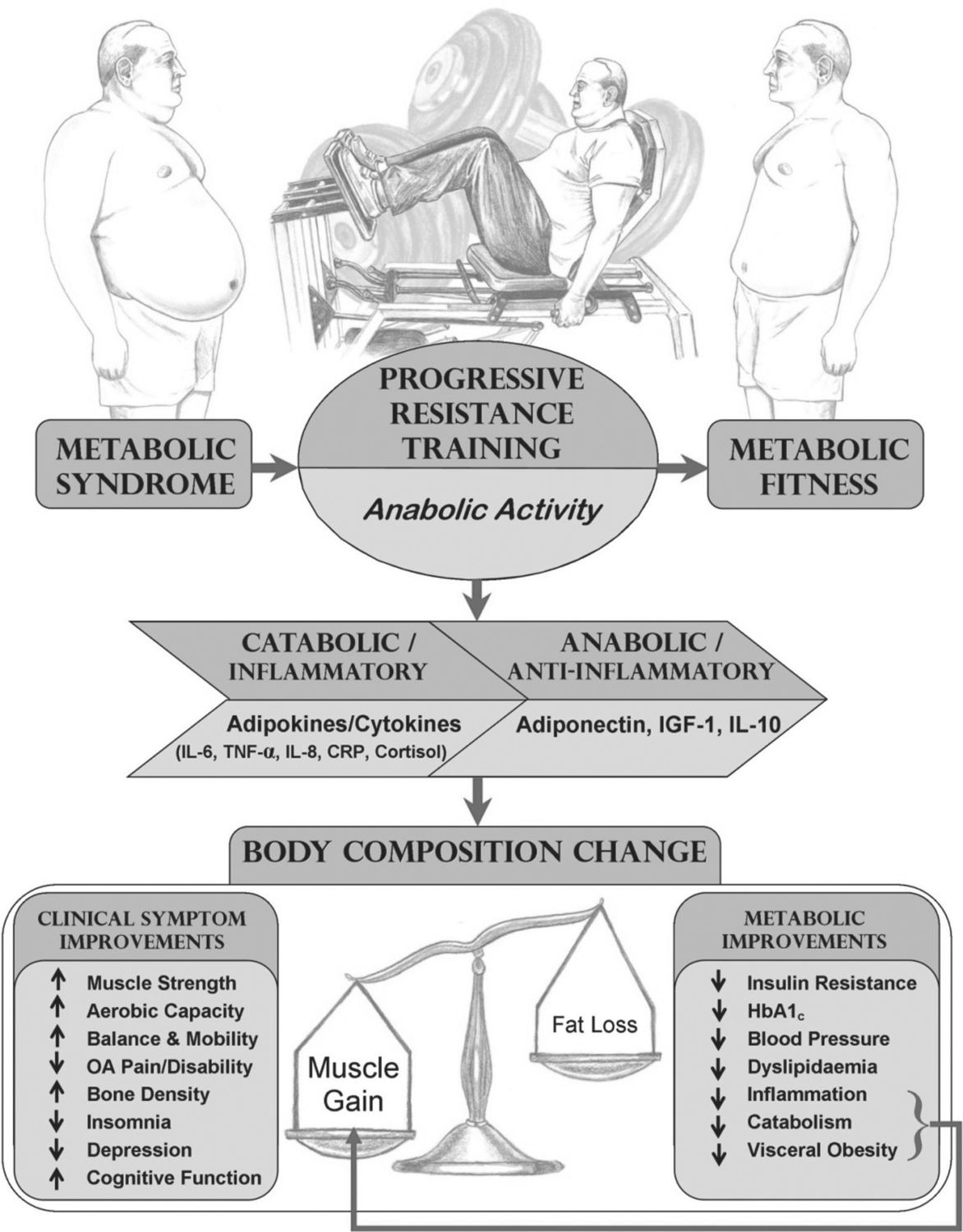
Figure 2. Schematic representation of PRT and its role in metabolic fitness. Legend: OA osteoarthritis; HbA1c glycosylated haemoglobin.
In summary, because of its manifold benefits, physical activity has been described as an important ‘medicine’ for the treatment of type 2 diabetes [108]. Moreover, the non-pharmacological nature of physical activity and the low cost of exercise programs also 287makes this intervention appealing [97]. Nevertheless, physical activity is still underutilised in clinical settings to manage diabetes [60, 109, 110, 111].
Recommendations for diabetes management
Individually tailored and structured physical activity programs are important in the management of diabetes [110]. For instance, the patient’s age, aerobic fitness, muscle strength, body composition, previous level of physical activity, timing and doses of insulin and oral hypoglycaemic agents, and comorbidities and diabetic complications should be considered in exercise prescriptions for individuals with type 2 diabetes [43, 58]. In particular, co-existent osteoarthritis, cardiovascular disease, orthostatic hypotension, peripheral vascular disease, and peripheral neuropathy, all common in type 2 diabetes, will influence the modality and intensity of exercise, the need for supervision, and other specific recommendations.
To improve glycaemic control, support weight maintenance, and reduce the risk of cardiovascular disease, the American Diabetes Association (ADA) and the Amercian College of Sports Medicine (ACSM) recommends individuals with type 2 diabetes to engage in at least 150 minutes per week of moderate-intensity physical activity (40%–60% of VO2max, which equates to 50%–70% of the maximum heart rate) or at least 60 minutes per week of vigorous aerobic exercise (>60% of VO2max or >70% of maximum heart rate) [43, 112]. Engaging in moderate to vigorous aerobic and/or resistance training yields greater benefits in the reduction of cardiovascular risk than lower volumes of physical activity [43], and an increased volume of activity is required for weight loss [43, 51]. Depending on the duration and intensity of physical activity the increased insulin sensitivity lasts for 24 to 72 hours after an activity session [113]. This underlines the importance of regular physical activity and therefore it is recommended that individuals with type 2 diabetes should not have more than two consecutive days without physical activity [43, 112].
Physical activity recommendations for adults with type 2 diabetes traditionally focused on aerobic activities, such as walking [114]. However, recent evidence has highlighted the effects of resistance training in addition to aerobic activities. Based on the substantial evidence that highlights the benefits of resistance and aerobic training, a combination of both exercise modalities appears optimal [76]. This is also stated in the recommendations for the general population of the American College of Sports Medicine, the American Diabetes Association, the American Heart Association, and the US Department of Health and Human Services that highlight the importance of incorporating resistance training into physical activity, especially for older adults [1, 3, 7, 112].
Pre-exercise screening should be considered for patients with type 2 diabetes. Screening should consider retinal disease, osteoarthritis, orthostatic hypotension, occult cardiovascular disease, peripheral vascular disease, peripheral neuropathy and diabetic foot disease, depression, cognitive impairment and gait and balance disorders to reduce potential risks and guide the specific exercise recommendations relevant to each individual.288
There have been some concerns among medical practitioners about health risks associated with high-intensity resistance training for older adults. Particularly, these concerns are about acute elevation of blood pressure and an increase in risk of stroke, myocardial infarction, and retinal haemorrhage. However, there is no evidence that resistance training actually increases the risk of such events [43]. In fact, chronic resistance training is associated with lower blood pressure [77, 115] and lower risk of cardiovascular events and mortality [116].
Physical activity and diabetes complications
People with diabetes have at least twice the risk of incident and fatal cardiovascular events compared to the general population [117, 118, 119]. Furthermore, people with diabetes with low cardio-respiratory fitness have a higher risk of overall mortality than those with higher fitness levels [120]. Physical activity improves fitness and reduces the risk of cardiovascular disease substantially, which makes an active lifestyle particularly important for people with diabetes [74].
Additionally, diabetes is associated with other medical comorbidity, including retinopathy, peripheral neuropathy, orthostatic hypotension, mobility impairment, osteoarthritis, peripheral vascular disease and renal disease. These can limit exercise capacity among patients with diabetes. Therefore, it is of particular importance for people with diabetes in the early stages to commence regular exercise [121]. Specific exercise regimens may be required in the presence of diabetic complications. For instance, patients with peripheral neuropathy may benefit from non-weight-bearing activities, such as resistance training, swimming or cycling [43]. Among patients with autonomic neuropathy or receiving betablockers, perceived exertion rather than the heart rate should be used to adjust the intensity of physical activity [109], and pre-exercise cardiac testing should be carried out before embarking on vigorous activity regimens in anyone with elevated cardiovascular risk, which includes most people with type 2 diabetes [88].
There are no known adverse effects of either resistance or aerobic training on vision or the progression of diabetic retinopathy. However, for patients with proliferative or severe non-proliferative retinopathy, moderate physical activity is recommended as vigorous exercise could potentially trigger vitreous haemorrhage or retinal detachment [43].
Blood glucose monitoring may be important before, during and after prolonged exercise. Acute bouts of exercise may require adjustment of medications on exercise days to prevent hypoglycaemia, the latter which may otherwise occur during exercise or many hours afterwards including nocturnally. Exercise may be timed for the post prandial peak in blood glucose; this will reduce the risk of exercise-related hypoglycaemia as well as reduce post-prandial hyperglycaemia and hyperinsulinemia [43, 122].
Challenges in implementation
There is substantial evidence for the health benefits of physical activity and exercise in the management of type 2 diabetes. However, as reported in studies from the US [123], Canada [59, 124] and Australia [110] the proportion of people with type 2 diabetes meeting the minimal physical activity recommendations is significantly lower than in the age-matched 289general population. An Australian population study reported significant gaps in physical activity recommendations and uptake as part of widespread diabetes management [110].
The principles of behaviour change are needed to encourage physical activity in the treatment of diabetes [59, 91, 111, 125]. Factors that facilitate or impede aerobic or resistance training need to be identified and addressed in clinical counselling. For example, older adults with diabetic complications may have difficulties engaging in more vigorous aerobic activities, and resistance training might be a better option. A frequent barrier is program costs, or the cost of resistance training equipment, and the daunting prospects of activity among sedentary individuals [76]. Since it is difficult to provide ongoing one-on-one supervision in resistance or aerobic training, understanding the maintenance of skills is required in the transition from supervised to independent training. Some resistance training interventions show long-term effects on muscle strength and body composition, but not on glycaemic control [114, 126].
Conclusion
Physical activity and exercise are effective in both prevention and treatment of diabetes, metabolic syndrome and cardiovascular disease. Both aerobic and resistance training have important roles in diabetes prevention and treatment, and the choice depends upon patient preferences, the need for supervision and the availability of exercise facilities. Higher doses and intensities of exercise are generally more effective, although there is benefit in starting at low to moderate levels of exercise intensity and volume, in order to reduce high dropout rates and prevent overuse injuries [75]. For some patients, exercise regimens should commence with moderate-intensity physical activities such as brisk walking [127, 128]. High volumes of exercise and incidental physical activity are required for weight loss, but activity can produce losses of the metabolically critical visceral fat compartment without overall change in body weight, and this can be of substantial benefit. On account of the acute bout effects, exercise frequency should be at least three days per week or more.
In summary, exercise is as potent as oral hypoglycaemic agents for glucose homeostasis, does not cause weight gain like insulin and oral agents, and provides additional benefits for fitness, functional independence, body composition, and vascular comorbidities which cannot be gained with pharmacologic or nutritional treatment alone. Thus, it should be seen as core to the prevention and treatment of type 2 diabetes, rather than an optional additional management strategy.
References
1. US Department of Health and Human Services (2008). 2008 physical activity guidelines for Americans. Washington, DC: US Department of Health and Human Services.
2. US Department of Health and Human Services (1996). Physical activity and health: a report of the surgeon general. Atlanta: Centers for Disease Control and Prevention.290
3. Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD & Bauman A (2007). Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Medicine and Science in Sports and Exercise, 39(8): 1423–34.
4. Caspersen CJ, Powell KE & Christenson GM (1985). Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Reports, 100(2): 126–31.
5. Thompson PD, Buchner D, Pina IL, Balady GJ, Williams MA, Marcus BH, Berra K, Blair S, Costa F, Franklin B, Fletcher G, Gordon N, Pate R, Rodriguez B, Yancy A & Wenger N (2003). Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity). Circulation, 107(24): 3109–16.
6. Hassinen M, Lakka TA, Hakola L, Savonen K, Komulainen P, Litmanen H, Kiviniemi V, Kouki R, Heikkilá H & Rauramaa R (2010). Cardiorespiratory fitness and metabolic syndrome in older men and women: the dose responses to exercise training (DR’s EXTRA) study. Diabetes Care, 33(7): 1655–57.
7. Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, Macera CA & Casteneda-Sceppa C (2007). Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Medicine and Science in Sports and Exercise, 39(8): 1435–45.
8. Bauman A, Bull F, Chey T, Craig CL, Ainsworth BE, Sallis JF, Bowles HR, Hagströmer M, Sjöström M, Pratt M & The IPS Group (2009). The international prevalence study on physical activity: results from 20 countries. International Journal of Behavioral Nutrition and Physical Activity, 6(1): 21.
9. Sjöström M, Oja P, Hagströmer M, Smith B & Bauman AE (2006). Health-enhancing physical activity across European Union countries: the Eurobarometer study. Journal of Public Health, 14(5): 291–300.
10. Stamatakis E, Ekelund U & Wareham NJ (2007). Temporal trends in physical activity in England: the health survey for England 1991 to 2004. Preventive Medicine, 45(6): 416–23.
11. Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T & McDowell M (2008). Physical activity in the United States measured by accelerometer. Medicine and Science in Sports and Exercise, 40(1): 181–88.
12. Asia-Pacific Physical Activity Network (2008). Regional physical activity prevalence in the Asia-Pacific region. Sydney: Asia-Pacific Physical Activity Network.
13. Chau J, Smith BJ, Bauman A, Merom D, Eyeson-Annan M, Chey T & Farrell L (2008). Recent trends in physical activity in New South Wales: is the tide of inactivity turning? Australian and New Zealand Journal of Public Health, 32(1): 82–5.
14. Zimmet P (2000). Globalization, coca-colonization and the chronic disease epidemic: can the doomsday scenario be averted? Journal of Internal Medicine, 247(3): 301–10.
15. LaMonte MJ, Blair SN & Church TS (2005). Physical activity and diabetes prevention. Journal of Applied Physiology, 99(3): 1205–13.
16. Hu G, Lakka TA & Tuomilehto J (2009). Physical activity, fitness, and the prevention of type 2 291diabetes. In IM Lee, S Blair, J Manson & RS Paffenbarger Jr (Eds). Epidemiologic methods in physical activity studies (pp 201–24). New York: Oxford University Press.
17. Chandran M, Phillips SA, Ciaraldi T & Henry RR (2003). Adiponectin: more than just another fat cell hormone? Diabetes Care, 26(8): 2442–50.
18. Pittas AG, Joseph NA & Greenberg AS (2004). Adipocytokines and insulin resistance. Journal of Clinical Endocrinology and Metabolism, 89(2): 447–52.
19. Simpson KA & Singh MA (2008). Effects of exercise on adiponectin: a systematic review. Obesity, 16(2): 241–56.
20. Weinstein AR, Sesso HD, Min Lee I, Cook NR, Manson JE, Buring JE & Gaziano JM (2004). Relationship of physical activity vs body mass index with type 2 diabetes in women. Journal of the American Medical Association, 292(10): 1188–94.
21. James SA, Jamjoum L, Raghunathan TE, Strogatz DS, Furth ED & Khazanie PG (1998). Physical activity and NIDDM in African-Americans: the Pitt County study. Diabetes Care, 21(4): 555–62.
22. Hu FB, Sigal RJ, Rich-Edwards JW, Colditz GA, Solomon CG, Willett WC, Speizer FE & Manson JE (1999). Walking compared with vigorous physical activity and risk of type 2 diabetes in women: a prospective study. Journal of the American Medical Association, 282(15): 1433–39.
23. Orchard TJ, Temprosa M, Goldberg R, Haffner S, Ratner R, Marcovina S, Fowler S & The Diabetes Prevention Program Research Group (2005). The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Annals of Internal Medicine, 142(8): 611–19.
24. Dempsey JC, Butler CL & Williams MA (2005). No need for a pregnant pause: physical activity may reduce the occurrence of gestational diabetes mellitus and preeclampsia. Exercises and Sport Science Reviews, 33(3): 141–49.
25. Hegaard HK, Pedersen BK, Nielsen BB & Damm P (2007). Leisure time physical activity during pregnancy and impact on gestational diabetes mellitus, pre-eclampsia, preterm delivery and birth weight: a review. Acta Obstetricia et Gynecologica, 86(11): 1290–96.
26. Jeon CY, Lokken RP, Hu FB & van Dam RM (2007). Physical activity of moderate intensity and risk of type 2 diabetes. Diabetes Care, 30(3): 744–52.
27. Healy GN, Dunstan DW, Salmon J, Cerin E, Shaw JE, Zimmet PZ & Owen N (2007). Objectively measured light-intensity physical activity is independently associated with 2-h plasma glucose. Diabetes Care, 30(6): 1384–89.
28. Hamilton MT, Healy GN, Dunstan DW, Zderic TW & Owen N (2008). Too little exercise and too much sitting: inactivity physiology and the need for new recommendations on sedentary behavior. Current Cardiovascular Risk Reports, 2(4): 292–98.
29. Owen N, Bauman A & Brown W (2009). Too much sitting: a novel and important predictor of chronic disease risk? British Journal of Sports Medicine, 43(2): 81–83.
30. Healy GN, Dunstan DW, Salmon J, Cerin E, Shaw JE, Zimmet PZ & Owen N (2008). Breaks in sedentary time: beneficial associations with metabolic risk. Diabetes Care, 31(4): 661–66.292
31. Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC & Rimm EB (2001). Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Archives of Internal Medicine, 161(12): 1542–48.
32. Hu FB, Li TY, Colditz GA, Willett WC & Manson JE (2003). Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. Journal of the American Medical Association, 289(14): 1785–91.
33. Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, Hu ZX, Lin J, Xiao JZ, Cao HB, Liu PA, Jiang XG, Jiang YY, Wang JP, Zheng H, Zhang H, Bennett PH & Howard BV (1997). Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: the Da Qing IGT and Diabetes Study. Diabetes Care, 20(4): 537–44.
34. Li G, Zhang P, Wang J, Gregg EW, Yang W, Gong Q, Li H, Jiang Y, An Y, Shuai Y, Zhang B, Zhang J, Thompson TJ, Gerzoff RB, Roqlic G, Hu Y & Bennett PH (2008). The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. The Lancet, 371(9626): 1783–89.
35. Diabetes Prevention Program Research Group (2002). Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New England Journal of Medicine, 346(6): 393–403.
36. Diabetes Prevention Program Research Group (2009). 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet, 374(9702): 1677–86.
37. Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Aunola S, Cepaitis Z, Moltchanov V, Hakumäki M, Mannelin M, Martikkala V, Sundvall J & Uusitupa M (2001). Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. New England Journal of Medicine, 344(18): 1343–50.
38. Lindström J, Ilanne-Parikka P, Peltonen M, Aunola S, Eriksson JG, Hemiö K, Hämäläinen H, Härkönen P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Mannelin M, Paturi M, Sundvall J, Valle TT, Uusitupa M & Tuomilehto J (2006). Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet, 368(9548): 1673–79.
39. Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD & Vijay V (2006). The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia, 49(2): 289–97.
40. Eriksson KF & Lindgarde F (1991). Prevention of type 2 (non-insulin-dependent) diabetes mellitus by diet and physical exercise: the 6-year Malmo feasibility study. Diabetologia, 34(12): 891–98.
41. Lindahl B, Nilssön TK, Borch-Johnsen K, Røder ME, Söderberg S, Widman L, Johnson O, Hallmans G & Jansson JH (2009). A randomized lifestyle intervention with 5-year follow-up in subjects with impaired glucose tolerance: pronounced short-term impact but long-term adherence problems. Scandinavian Journal of Public Health, 37(4): 434–42.
42. Kosaka K, Noda M & Kuzuya T (2005). Prevention of type 2 diabetes by lifestyle intervention: a Japanese trial in IGT males. Diabetes Research and Clinical Practice, 67(2): 152–62.293
43. Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C & White RD (2006). Physical activity/exercise and type 2 diabetes: a consensus statement from the American Diabetes Association. Diabetes Care, 29(6): 1433–38.
44. Orozco LJ, Buchleitner AM, Gimenez-Perez G, Figuls MRI, Richter B & Mauricio D (2008). Exercise or exercise and diet for preventing type 2 diabetes mellitus. Cochrane Database of Systematic Reviews, 3: CD003054.
45. Yates T, Khunti K, Bull F, Gorely T & Davies MJ (2007). The role of physical activity in the management of impaired glucose tolerance: a systematic review. Diabetologia, 50(6): 1116–26.
46. Montoye HJ, Kemper HCG, Saris WHM & Washburn RA (1996). Measuring physical activity and energy expenditure. Champaign: Human Kinetics.
47. Carnethon MR, Gidding SS, Nehgme R, Sidney S, Jacobs DR, Jr & Liu K (2003). Cardiorespiratory fitness in young adulthood and the development of cardiovascular disease risk factors. Journal of the American Medical Association, 290(23): 3092–100.
48. Sawada SS, Min Lee I, Naito H, Noguchi J, Tsukamoto K, Muto T, Higaki Y, Tanaka H & Blair SN (2010). Long-term trends in cardiorespiratory fitness and the incidence of type 2 diabetes. Diabetes Care, 33(6): 1353–57.
49. Sieverdes JC, Sui X, Lee DC, Church TS, McClain A, Hand GA & Blair SN (2010). Physical activity, cardiorespiratory fitness and the incidence of type 2 diabetes in a prospective study of men. British Journal of Sports Medicine, 44(4): 238–44.
50. Kesaniemi YK, Danforth E, Jr, Jensen MD, Kopelman PG, Lefebvre P & Reeder BA (2001). Dose-response issues concerning physical activity and health: an evidence-based symposium. Medicine and Science in Sports and Exercise, 33(Suppl. 6): S351–58.
51. Jakicic JM, Otto AD, Polzien K & Davis K (2009). Physical activity and weight control. In I Min Lee, S Blair, J Manson & RS Paffenbarger Jr, (Eds). Epidemiologic methods in physical activity research (pp225–45). New York: Oxford University Press.
52. Hill JO & Wyatt HR (2005). Role of physical activity in preventing and treating obesity. Journal of Applied Physiology, 99(2): 765–70.
53. Jakicic JM, Clark K, Coleman E, Donnelly JE, Foreyt J, Melanson E, Volek J & Volpe SL (2001). American College of Sports Medicine position stand: appropriate intervention strategies for weight loss and prevention of weight regain for adults. Medicine and Science in Sports and Exercise, 33(12): 2145–56.
54. van der Ploeg HP, Smith BJ, Stubbs T, Vita P, Holford R & Bauman AE (2007). Physical activity promotion: are GPs getting the message? Australian Family Physician, 36(10): 871–74.
55. Duncan GE, Anton SD, Sydeman SJ, Newton RL Jr, Corsica JA, Durning PE, Ketterson TU, Martin AD, Limacher MC & Perri MG (2005). Prescribing exercise at varied levels of intensity and frequency: a randomized trial. Archives of Internal Medicine, 165(20): 2362–69.
56. Bauman A, Murphy N & Lane A (2009). The role of community programmes and mass events in promoting physical activity to patients. British Journal of Sports Medicine, 43(1): 44–46.
57. Rosenberg DE, Jabbour SA & Goldstein BJ (2005). Insulin resistance, diabetes and cardiovascular risk: approaches to treatment. Diabetes Obesity & Metabolism, 7(6): 642–53.294
58. Praet SFE & van Loon LJC (2007). Optimizing the therapeutic benefits of exercise in type 2 diabetes. Journal of Applied Physiology, 103(4): 1113–20.
59. Boudreau F & Godin G (2009). Understanding physical activity intentions among French Canadians with type 2 diabetes: an extension of Ajzen’s theory of planned behaviour. International Journal of Behavioral Nutrition and Physical Activity, 6(110): 35.
60. Kirk A, Barnett J, Leese G & Mutrie N (2009). A randomized trial investigating the 12-month changes in physical activity and health outcomes following a physical activity consultation delivered by a person or in written form in type 2 diabetes: Time2Act. Diabetic Medicine, 26(3): 293–301.
61. Plotnikoff RC, Karunamuni ND, Johnson JA, Kotovych M & Svenson LW (2008). Health-related behaviours in adults with diabetes: associations with healthcare utilization and costs. Canadian Journal of Public Health, 99(3): 227–31.
62. Boule NG, Kenny GP, Haddad E, Wells GA & Sigal RJ (2003). Meta-analysis of the effect of structured exercise training on cardiorespiratory fitness in type 2 diabetes mellitus. Diabetologia, 46(8): 1071–81.
63. Tresierras MA & Balady GJ (2009). Resistance training in the treatment of diabetes and obesity: mechanisms and outcomes. Journal of Cardiopulmonary Rehabilitation & Prevention, 29(2): 67–75.
64. Sigal RJ, Kenny GP, Boule NG, Wells GA, Prud’homme D, Fortier M, Reid RD, Tulloch H, Coyle D, Phillips P, Jennings A & Jaffey J (2007). Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Annals of Internal Medicine, 147(6): 357–69.
65. Marcus RL, Smith S, Morrell G, Addison O, Dibble LE, Wahoff-Stice D & LaStayo PC (2008). Comparison of combined aerobic and high-force eccentric resistance exercise with aerobic exercise only for people with type 2 diabetes mellitus. Physical Therapy, 88(11): 1345–54.
66. Wang Y, Simar D & Fiatarone Singh MA (2009). Adaptations to exercise training within skeletal muscle in adults with type 2 diabetes or impaired glucose tolerance: a systematic review. Diabetes Metabolism Research and Reviews, 25(1): 13–40.
67. Yang H, Youm YH, Vandanmagsar B, Ravussin A, Gimble JM, Greenway F, Stephens JM, Mynatt RL & Dixit VD (2010). Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. Journal of Immunology, 185(3): 1836–45.
68. Olefsky JM & Glass CK (2010). Macrophages, inflammation, and insulin resistance. Annual Review of Physiology, 72: 219–46.
69. Mourier A, Gautier JF, De Kerviler E, Bigard AX, Villette JM, Garnier JP, Duvallet A, Guezennec CY & Cathelineau G (1997). Mobilization of visceral adipose tissue related to the improvement in insulin sensitivity in response to physical training in NIDDM. Effects of branched-chain amino acid supplements. Diabetes Care, 20(3): 385–91.
70. Kirwan JP, Solomon TP, Wojta DM, Staten MA & Holloszy JO (2009). Effects of 7 days of exercise training on insulin sensitivity and responsiveness in type 2 diabetes mellitus. American Journal of Physiology, Endocrinology and Metabolism, 297(1): E151–56.295
71. Yokoyama H, Emoto M, Fujiwara S, Motoyama K, Morioka T, Koyama H, Shoji T, Inaba M & Nishizawa Y (2004). Short-term aerobic exercise improves arterial stiffness in type 2 diabetes. Diabetes Research & Clinical Practice, 65(2): 85–93.
72. Sykes K, Yeung TLV & Ko GTC (2004). A 12-week prospective randomized controlled trial to investigate the effects of aerobic training on type 2 diabetes patients. American Journal of Recreation Therapy, 3(3): 36–42.
73. Ribeiro IC, Iborra RT, Neves MQ, Lottenberg SA, Charf AM, Nunes VS, Negrão CE, Nakandakare ER, Quintão EC & Passarelli M (2008). HDL atheroprotection by aerobic exercise training in type 2 diabetes mellitus. Medicine and Science in Sports and Exercise, 40(5): 779–86.
74. Jakicic JM, Jaramillo SA, Balasubramanyam A, Bancroft B, Curtis JM, Mathews A, Pereira M, Regensteiner JG & Ribisl PM (2009). Effect of a lifestyle intervention on change in cardiorespiratory fitness in adults with type 2 diabetes: results from the Look AHEAD Study. International Journal of Obesity, 33(3): 305–16.
75. Praet SF, van Rooij ES, Wijtvliet A, Boonman-de Winter LJ, Enneking T, Kuipers H, Stehouwer CD & van Loon LJ (2008). Brisk walking compared with an individualised medical fitness programme for patients with type 2 diabetes: a randomised controlled trial. Diabetologia, 51(5): 736–46.
76. Eves ND & Plotnikoff RC (2006). Resistance training and type 2 diabetes: considerations for implementation at the population level. Diabetes Care, 29(8): 1933–41.
77. Strasser B, Siebert U & Schobersberger W (2010). Resistance training in the treatment of the metabolic syndrome: a systematic review and meta-analysis of the effect of resistance training on metabolic clustering in patients with abnormal glucose metabolism. Sports Medicine, 40(5): 397–415.
78. Holten MK, Zacho M, Gaster M, Juel C, Wojtaszewski JF & Dela F (2004). Strength training increases insulin-mediated glucose uptake, GLUT4 content, and insulin signaling in skeletal muscle in patients with type 2 diabetes. Diabetes, 53(2): 294–305.
79. Ishii T, Yamakita T, Sato T, Tanaka S & Fujii S (1998). Resistance training improves insulin sensitivity in NIDDM subjects without altering maximal oxygen uptake. Diabetes Care, 21(8): 1353–55.
80. Misra A, Alappan NK, Vikram NK, Goel K, Gupta N, Mittal K & Bhatt SL (2008). Effect of supervised progressive resistance-exercise training protocol on insulin sensitivity, glycemia, lipids, and body composition in Asian Indians with type 2 diabetes. Diabetes Care, 31(7): 1282–87.
81. Dunstan DW, Daly RM, Owen N, Jolley D, De Courten M, Shaw J & Zimmett P (2002). High-intensity resistance training improves glycemic control in older patients with type 2 diabetes. Diabetes Care, 25(10): 1729–36.
82. Castaneda C, Layne JE, Munoz-Orians L, Gordon PL, Walsmith J, Foldvari M, Roubenoff R, Tucker KL & Nelson ME (2002). A randomized controlled trial of resistance exercise training to improve glycemic control in older adults with type 2 diabetes. Diabetes Care, 25(12): 2335–41.
83. Eriksson J, Taimela S, Eriksson K, Parviainen S, Peltonen J & Kujala U (1997). Resistance training in the treatment of non-insulin-dependent diabetes mellitus. International Journal of Sports Medicine, 18(4): 242–46.296
84. Honkola A, Forsen T & Eriksson J (1997). Resistance training improves the metabolic profile in individuals with type 2 diabetes. Acta Diabetologica, 34(4): 245–48.
85. Dunstan DW, Puddey IB, Beilin LJ, Burke V, Morton AR & Stanton KG (1998). Effects of a shortterm circuit weight training program on glycaemic control in NIDDM. Diabetes Research and Clinical Practice, 40(1): 53–61.
86. Maiorana A, O’Driscoll G, Goodman C, Taylor R & Green D (2002). Combined aerobic and resistance exercise improves glycemic control and fitness in type 2 diabetes. Diabetes Research and Clinical Practice, 56(2): 115–23.
87. Cuff DJ, Meneilly GS, Martin A, Ignaszewski A, Tildesley HD & Frohlich JJ (2003). Effective exercise modality to reduce insulin resistance in women with type 2 diabetes. Diabetes Care, 26(11): 2977–82.
88. Kavookjian J, Elswick BM & Whetsel T (2007). Interventions for being active among individuals with diabetes: a systematic review of the literature. The Diabetes Educator, 33(6): 962–88.
89. Singh NA, Stavrinos TM, Scarbek Y, Galambos G, Liber C & Fiatarone Singh MA (2005). A randomized controlled trial of high versus low intensity weight training versus general practitioner care for clinical depression in older adults. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 60(6): 768–76.
90. Perrig-Chiello P, Perrig WJ, Ehrsam R, Staehelin HB & Krings F (1998). The effects of resistance training on wellbeing and memory in elderly volunteers. Age and Ageing, 27(4): 469–75.
91. Plotnikoff RC (2006). Physical activity in the management of diabetes: population-based perspectives and strategies. Canadian Journal of Diabetes, 30(1): 52–62.
92. Ivy JL (1997). Role of exercise training in the prevention and treatment of insulin resistance and non-insulin-dependent diabetes mellitus. Sports Medicine, 24(5): 321–36.
93. Balducci S, Leonetti F, Di Mario U & Fallucca F (2004). Is a long-term aerobic plus resistance training program feasible for and effective on metabolic profiles in type 2 diabetic patients? Diabetes Care, 27(3): 841–42.
94. Snowling NJ & Hopkins WG (2006). Effects of different modes of exercise training on glucose control and risk factors for complications in type 2 diabetic patients: a meta-analysis. Diabetes Care, 29(11): 2518–27.
95. Fiatarone Singh M (2002). Exercise comes of age: rationale and recommendations for a geriatric exercise prescription. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 57(5): M262–M82.
96. Daly RM, Dunstan DW, Owen N, Jolley D, Shaw JE & Zimmet PZ (2005). Does high-intensity resistance training maintain bone mass during moderate weight loss in older overweight adults with type 2 diabetes? Osteoporosis International, 16(12): 1703–12.
97. Thomas DR, Elliott EJ & Naughton GA (2006). Exercise for type 2 diabetes mellitus. Cochrane Database of Systematic Reviews, 3: CD002968.
98. Boule NG, Haddad E, Kenny GP, Wells GA & Sigal RJ (2001). Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. Journal of the American Medical Association, 286(10): 1218–27.297
99. Khaw KT, Wareham N, Luben R, Bingham S, Oakes S, Welch A & Day N (2001). Glycated haemoglobin, diabetes, and mortality in men in Norfolk cohort of European Prospective Investigation of Cancer and Nutrition (EPIC-Norfolk). British Medical Journal, 322(7277): 15–18.
100. Baldi JC, Snowling N (2003). Resistance training improves glycaemic control in obese type 2 diabetic men. International Journal of Sports Medicine, 24(6): 419–23.
101. Lee SH, Park SA, Ko SH, Yim HW, Ahn YB, Yoon KH, Cha BY & Kwon HS (2010). Insulin resistance and inflammation may have an additional role in the link between cystatin C and cardiovascular disease in type 2 diabetes mellitus patients. Metabolism, 59(2): 241–46.
102. Wojtaszewski JF & Richter EA (2006). Effects of acute exercise and training on insulin action and sensitivity: focus on molecular mechanisms in muscle. Essays in Biochemistry, 42: 31–46.
103. Krook A, Holm I, Pettersson S & Wallberg-Henriksson H (2003). Reduction of risk factors following lifestyle modification programme in subjects with type 2 (non-insulin dependent) diabetes mellitus. Clinical Physiology and Functional Imaging, 23(1): 21–30.
104. Rubin RR, Gaussoin SA, Peyrot M, DiLillo V, Miller K, Wadden TA, West DS, Wing RR & Knowler WC (2010). Cardiovascular disease risk factors, depression symptoms and antidepressant medicine use in the Look AHEAD (Action for Health in Diabetes) clinical trial of weight loss in diabetes. Diabetologia, 53(8): 1581–89.
105. Wing RR, Rosen RC, Fava JL, Bahnson J, Brancati F, Gendrano IN, Kitabchi A, Schneider SH & Wadden TA (2010). Effects of weight loss intervention on erectile function in older men with type 2 diabetes in the Look AHEAD trial. Journal of Sexual Medicine, 7(1 Pt 1): 156–65.
106. Curtis JM, Horton ES, Bahnson J, Gregg EW, Jakicic JM, Regensteiner JG, Ribisl PM, Soberman JE, Stewart KJ & Espeland MA (2010). Prevalence and predictors of abnormal cardiovascular responses to exercise testing among individuals with type 2 diabetes: the Look AHEAD (Action for Health in Diabetes) study. Diabetes Care, 33(4): 901–07.
107. Albu JB, Heilbronn LK, Kelley DE, Smith SR, Azuma K, Berk ES, Pi-Sunyer FX, Ravussin E & Look AHEAD Adipose Research Group (2010). Metabolic changes following a 1-year diet and exercise intervention in patients with type 2 diabetes. Diabetes, 59(3): 627–33.
108. Tucker PS, Fisher-Wellman K & Bloomer RJ (2008). Can exercise minimize postprandial oxidative stress in patients with type 2 diabetes? Current Diabetes Review, 4(4): 309–19.
109. Albright A, Franz M, Hornsby G, Kriska A, Marrero D, Ullrich I & Verity LS (2000). American College of Sports Medicine position stand: exercise and type 2 diabetes. Medicine and Science in Sports and Exercise, 32(7): 1345–60.
110. Neville L & Bauman A (2004). Self-reported risk factors and management strategies used by people with diabetes mellitus identified from the 1997 and 1998 NSW Health Surveys. NSW Public Health Bulletin, 15(4): 57–62.
111. Kirk AF, Barnett J & Mutrie N (2007). Physical activity consultation for people with type 2 diabetes. Evidence and guidelines. Diabetic Medicine, 24(8): 809–16.
112. American College of Sports Medicine and American Diabetes Association (2010). Exercise and 298type 2 diabetes. American College of Sports Medicine and the American Diabetes Association: joint position statement. Medicine and Science in Sports and Exercise, 42(12): 2282–303.
113. Wallberg-Henriksson H, Rincon J & Zierath JR (1998). Exercise in the management of non-insulin-dependent diabetes mellitus. Sports Medicine, 25(1): 25–35.
114. Dunstan DW, Daly RM, Owen N, Jolley D, Vulikh E, Shaw J & Zimmet P (2005). Home-based resistance training is not sufficient to maintain improved glycemic control following supervised training in older individuals with type 2 diabetes. Diabetes Care, 28(1): 3–9.
115. Fagard RH & Cornelissen VA (2007). Effect of exercise on blood pressure control in hypertensive patients. European Journal of Cardiovascular Prevention & Rehabilitation, 14(1): 12–17.
116. Tanasescu M, Leitzmann MF, Rimm EB, Willett WC, Stampfer MJ & Hu FB (2002). Exercise type and intensity in relation to coronary heart disease in men. Journal of the American Medical Association, 288(16): 1994–2000.
117. Meltzer S, Leiter L, Daneman D, Gerstein HC, Lau D, Ludwig S, Yale JF, Zinman B, Lillie D & Steering and Expert Committees (1998). 1998 clinical practice guidelines for the management of diabetes in Canada. Canadian Diabetes Association. Canadian Medical Association Journal, 159(Suppl. 8): S1–29.
118. Skerrett PJ & Manson JE (2002). Reduction in risk of coronary heart disease and diabetes. In N Ruderman, JT Devlin, SH Schneider & A Kriska (Eds). Handbook of exercise in diabetes (pp 155–81). Alexandria, VA: American Diabetes Association.
119. Buse JB, Ginsberg HN, Bakris GL, Clark NG, Costa F, Eckel R, Fonseca V, Gerstein HC, Grundy S, Nesto RW, Pignone MP, Plutzky J, Porte D, Redberg R, Stitzel KF, Stone NJ, American Heart Association & American Diabetes Association (2007). Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care, 30(1): 162–72.
120. Church TS, Cheng YJ, Earnest CP, Barlow CE, Gibbons LW, Priest EL & Blair SN (2004). Exercise capacity and body composition as predictors of mortality among men with diabetes. Diabetes Care, 27(1): 83–88.
121. Constantini N, Harman-Boehm I & Dubnov G (2005). Exercise prescription for diabetics: more than a general recommendation. Harefuah, 144(10): 717–23, 50.
122. Hayes C & Kriska A (2008). Role of physical activity in diabetes management and prevention. Journal of the American Dietetic Association, 108(4): S19–S23.
123. Nelson KM, Reiber G & Boyko EJ (2002). Diet and exercise among adults with type 2 diabetes: findings from the third national health and nutrition examination survey (NHANES III). Diabetes Care, 25(10): 1722–28.
124. Plotnikoff RC, Taylor LM, Wilson PM, Courneya KS, Sigal RJ, Birkett N, Raine K & Svenson LW (2006). Factors associated with physical activity in Canadian adults with diabetes. Medicine and Science in Sports and Exercise, 38(8): 1526–34.
125. Plotnikoff R, Lippke S, Courneya K, Birkett N & Sigal R (2008). Physical activity and social 299cognitive theory: a test in a population sample of adults with type 1 or type 2 diabetes. Applied Psychology: An International Review, 57(4): 628–43.
126. Plotnikoff RC, Eves N, Jung M, Sigal RJ, Padwal R & Karunamuni N (2010). Multicomponent, home-based resistance training for obese adults with type 2 diabetes: a randomized controlled trial. International Journal of Obesity, 34(12): 1733–41.
127. Eyler AA, Brownson RC, Bacak SJ & Housemann RA (2003). The epidemiology of walking for physical activity in the United States. Medicine and Science in Sports and Exercise, 35(9): 1529–36.
128. Li F, Fisher KJ, Brownson RC & Bosworth M (2005). Multilevel modelling of built environment characteristics related to neighbourhood walking activity in older adults. Journal of Epidemiology and Community Health, 59(7): 558–64.