8
One Health surveillance: monitoring health risks at the human–animal–environment interface
One Health surveillance
Effective surveillance and response relies on networks linking diverse information sources (Gresham et al. 2013). In the context of One Health, this can include gathering disease and population statistics from humans, domestic and wild animals, coupled with data on the environment and climate, and developing networks to distribute information via multiple stakeholder groups so that appropriate actions can be taken. The International Society for Disease Surveillance (ISDS) defines One Health surveillance as ‘the collaborative, on-going, systematic collection and analysis of data from multiple domains (at local, national and global levels) to detect health-related events and produce information which leads to actions aimed at attaining optimal health for people, animals, and the environment’ (ISDS 2017). This builds on the World Health Organization (WHO) and World Organisation for Animal Health (OIE, after the original French name Office International des Epizooties) definitions for public health and animal health surveillance, respectively.
Box 8.1 presents a case study of a disease (Rift Valley fever, RVF) that warrants a One Health approach to surveillance. Experience with RVF outbreaks has led to formulation of frameworks for collaboration and coordination across agencies for this particular disease, as will be discussed later in this chapter. In the absence of such frameworks, response teams managing diseases at the human–animal–environment interface are often confronted with the following questions:
- What are the potential risks to human health?
- What are the potential risks to animal health and trade?
- How is the outbreak first recognised and notified to agencies?
- What factors determine the make-up of the response team?
- Which agency assumes the role of coordination and lead?
- Who covers expenses including laboratory testing?
- Which agency is responsible for ongoing surveillance?
- Who is responsible for providing information to the media and managing public awareness?
- What are the environmental impacts and how will they be managed?
In an ideal situation, one government agency would cover all aspects of health and develop an overarching surveillance system with an integrated outbreak response unit; one that complies with a universal investigation protocol. However, there are many obstacles to overcome before this model becomes the norm. For now, it is necessary for agencies from disparate backgrounds and with different objectives to collaborate effectively when confronted with emerging threats.
In this chapter, we consider the range of surveillance options as well as challenges to monitoring disease at the human–animal–environment interface. In the first section, we compare and contrast the different sectoral approaches to disease surveillance in humans and other animals. Practitioners intending to collect, aggregate and/or synthesise data generated from such programs need to understand the methods and motivations behind surveillance in each sector as this is crucial for conceiving new approaches to monitor health risks at the interface. In the second section, we discuss challenges and opportunities to achieving One Health surveillance. We then explore how One Health surveillance is being implemented across a range of applications in the third section. We conclude by recommending ways to enhance One Health surveillance.
Sectoral approaches to surveillance
At its core – and irrespective of whether it is for human or animal populations – disease surveillance is the process of gathering, interpreting and sharing intelligence on the occurrence of disease and other health outcomes in populations. Such systems typically comprise a system for mandatory (required by law) reporting of ‘notifiable’ conditions to the relevant government authority(ies). The objectives of surveillance are broadly similar across sectors, namely:
- monitoring disease trends, such as estimating the magnitude or geographic distribution of a health problem
- detecting outbreaks or epidemics, including emerging diseases
- generating hypotheses regarding the aetiology and transmission pathways of disease outbreaks
- supporting decision-making regarding research priorities and healthcare/disease control programming
- directing and evaluating control strategies
- demonstrating absence of (or ‘freedom from’) disease.
The last objective provides assurances for trade and tourism. This is crucial in the livestock sector since animal health surveillance data are used in import risk analysis (IRA). Surveillance – specifically proof of freedom surveys1 – permits countries or zones within a country to be declared free of disease for trade purposes. Peeler, Reese, and Thrush (2015) and the OIE handbooks on import risk analysis for animals and animal products (Bruckner et al. 2010; Murray et al. 2010) provide an overview of IRA for those wanting more information. While this last objective receives less attention in public health, monitoring of disease-free status can be useful for tourism; for example, identifying areas free of malaria.
General approaches to disease surveillance
Methods for disease surveillance are similar across human and animal health, although triggering events, disease priorities and reporting lines differ markedly between sectors (Table 8.1). It is our impression that – compared to public health surveillance – animal health surveillance encompasses a broader range of activities, such as participatory disease surveillance (see Box 8.2) and structured population-based surveys. The latter includes prevalence surveys and proof of freedom surveys which aim to make statistical inferences about the frequency (e.g. prevalence) or absence of disease, respectively, in the total animal population under surveillance. Such investigations – typically carried out in defined populations and within a discrete period of time – are commonly classified as research activity in public health sectors. The emerging use of whole genome sequencing (WGS) as a tool to monitor strains is becoming accepted practice in the public health sector and stretches traditional understanding of surveillance into the area of research (Kwong et al. 2015).
Traditional surveillance systems are designed to collect data on specific diseases (so-called indicator-based surveillance). Laboratory confirmation is usually required according to a prescribed criterion or ‘case definition’. This applies to human and animal diseases, with newly emerging disease risks added to the systems as indicated. To improve timeliness, notification of serious diseases (e.g. typhoid and measles in humans; foot-and-mouth disease and highly pathogenic avian influenza in livestock and poultry, respectively) is recommended to occur on suspicion and before laboratory confirmation, based on symptoms and signs consistent with disease rather than laboratory criteria. Some surveillance systems – such as those designed to support smallpox and polio eradication or to monitor trends in gastroenteritis and influenza-like illness – rely exclusively on clinical, and not laboratory case definitions. This is sometimes called ‘symptomatic surveillance’ or ‘syndromic surveillance’; although the latter term is also used to describe non-traditional data sources in disease surveillance (see below). Since laboratory confirmation is not required, this type of surveillance is particularly useful in resource-poor settings or as an early warning system since time to detection is more rapid (May et al. 2011)
Table 8.1. Properties of surveillance systems across human and animal health sectors.
|
|
Humans |
Terrestrial livestock |
Aquatic animals |
Wild animals |
Companion animals |
|---|---|---|---|---|---|
|
Intergovernmental organisation with mandate |
World Health Organization (WHO) |
World Organisation for Animal Health (OIE)2 |
World Organisation for Animal Health (OIE)2 |
World Organisation for Animal Health (OIE)2 [voluntary; effective 1993] |
None3 |
|
National agencies responsible for collating surveillance data |
Ministry representing health |
Ministry representing livestock/animal resources/agriculture |
Ministry representing fisheries |
Various. May OIE National Focal Point for Wildlife |
None3 |
|
Disease priorities |
Blood-borne diseases, gastro-intestinal diseases,1 quarantinable diseases, sexually transmitted infections, vaccine-preventable diseases, vector-borne diseases,1 and other zoonoses |
OIE-listed diseases (trade-sensitive diseases) |
OIE-listed diseases (trade-sensitive diseases) |
OIE-listed diseases (trade-sensitive diseases); non-listed diseases with conservation impacts |
No formal disease surveillance in most countries3 |
|
Triggering events |
Patient visits healthcare provider |
Farmer contacts veterinarian after noticing illness; livestock inspector/veterinarian detects disease at saleyard/abattoir |
Observation of unusual morbidity |
Observation of unusual morbidity |
Owner takes pet to veterinary clinic |
|
Principal stakeholders in surveillance system |
Patients, medical practitioners, laboratories, public health units |
Farmers, livestock handlers, veterinarians, laboratories |
Fishermen, veterinarians, laboratories |
Wildlife biologists, ecologists, hunters, wildlife managers, rehabilitators, conservation managers |
Animal owners, veterinarians, laboratories |
|
Common methods for data collection |
Passive surveillance for notifiable conditions; active surveillance; sentinel surveillance; syndromic surveillance; event-based surveillance |
Passive surveillance for notifiable conditions; abattoir inspection; structured population-based surveys; sentinel surveillance |
Autopsies and laboratory analysis |
Autopsies and laboratory analysis |
No formal disease surveillance in most countries3 |
|
1Many of these diseases have zoonotic origins. 2 Office International des Epizooties 3 OIE-listed diseases which occur in companion animals may be notified to the ministry representing livestock services/agriculture/fisheries. | |||||
Surveillance systems are traditionally classified as ‘passive’ or ‘active’, depending on how reporting is initiated. In passive surveillance systems, government jurisdictions receive reports from medical practitioners/ veterinarians and laboratories when a case is diagnosed. This is the most common type of surveillance for notifiable diseases in both public health and animal health sectors. Passive surveillance systems are low-input in terms of cost and labour but potentially suffer from under-reporting. It has become standard practice in some countries to provide automated electronic reporting directly from the laboratory with benefits of improved reporting completeness and reduced workload. In active surveillance systems, government agencies contact medical practitioners/veterinarians, laboratories, pharmacists and other providers to obtain data on cases. This is particularly useful during outbreak investigations as it heightens awareness and improves case ascertainment. Active surveillance is more time-consuming and costlier than passive reporting; however, it results in more complete reporting.
In the above examples of notifiable disease surveillance data are collected on the whole population; healthcare providers and laboratories report all diagnosed cases to government authorities. In some circumstances surveillance is performed by monitoring specific sites, providers or vectors/animals (so-called sentinel surveillance). Detection of the pathogen/disease in the sentinel population(s) is then taken to reflect the risk to the wider population. A common application is the use of sentinel chicken flocks to detect arboviral activity, such as West Nile virus in the United States and Murray Valley encephalitis virus in Australia. In the animal health sector, sentinel cattle herds are used to monitor the spread of bluetongue virus in Europe and Australia. Sentinel surveillance is discussed later in the chapter.
The advent of electronic data collection, increased computing power and the internet has generated interest in non-traditional data sources for disease surveillance (also called ‘syndromic surveillance’) motivated principally by the desire for earlier detection of outbreaks, that is, before laboratory information is available (Figure 8.1). By monitoring clinical (e.g. emergency department admissions) or non-clinical (e.g. over-the-counter prescription sales, school absenteeism) indicators in real-time, health departments can potentially detect early signs of an adverse health event (Henning 2004). Similar approaches have been developed in animal health and are reviewed by Dorea, Sanchez, and Revie (2011). Key challenges with this approach relate to the non-specificity of the signal and defining the threshold for alert against substantial background ‘noise’ (Henning 2004).
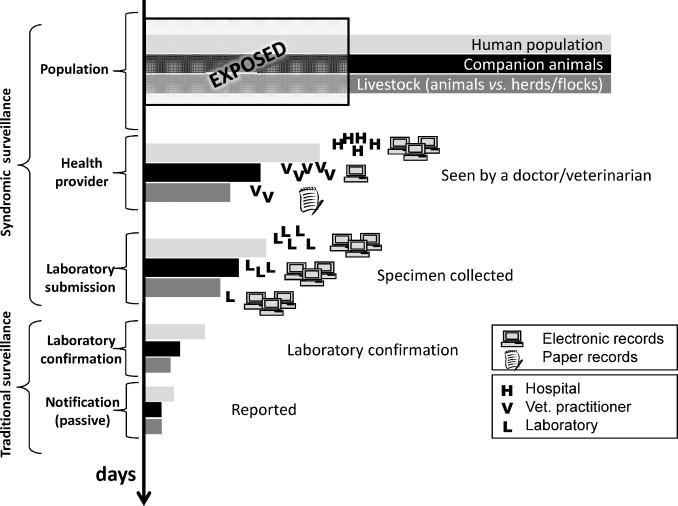
Figure 8.1 Schematic depicting the data sources used for traditional and syndromic surveillance systems. The Y axis represents the time to detection (longest = bottom). The X axis represents the (relative) number of cases detected by each approach. Adapted from Dorea, Sanchez and Revie (2011).
Indicator-based surveillance systems can be complemented by ‘event-based surveillance systems’. Under event-based surveillance, internet and media sources are scanned for information that may signal an unusual or emerging health threat. Signals may come from reports, rumours or other stories about illness in the community. Community members or news reporters may also pass on information that warrants investigation. Whereas indicator-based surveillance typically involves analysing and interpreting data collected in a standardised way and originating from within the human and animal healthcare systems, the focus of event-based surveillance is capturing, filtering and verifying non-standardised reports of potential health events from external sources. HealthMap (www.healthmap.org) is a good example of a comprehensive system combining a wide variety of indicator- and event-based data.
Disease surveillance in humans
The public health surveillance obligations of WHO member nations are described in the International Health Regulations (IHR). Early versions of these regulations focused on diseases such as smallpox, cholera, yellow fever and plague (Nuttall et al. 2014). However, with the emergence of severe acute respiratory syndrome (SARS) and its rapid spread around the world, the deficiencies of the regulations became obvious, necessitating their review. The current IHR (2005) require WHO members to develop and maintain adequate surveillance capacity to identify any event that constitutes a ‘public health emergency of international concern’ and to report these promptly to the WHO (WHO 2005). In 2014, the Global Health Security Agenda (www.ghsagenda.org) was launched to help countries achieve core capacities required by the IHR 2005, and is complemented on the animal health side by OIE’s Performance of Veterinary Services Pathway (see below). Surveillance is also important for tracking progress towards Sustainable Development Goal (SDG) 3, which sets targets for reducing the burden of HIV, tuberculosis, malaria, and other communicable diseases (http://bit.ly/2RH2Bi0). Aggregated data showing progress towards the SDGs are publicly available via the WHO Global Health Observatory (www.who.int/gho/en/).
Disease surveillance in animals
The OIE maintains a list of notifiable conditions which is reviewed annually. The current OIE list includes 117 terrestrial and aquatic animal diseases and infections,2 many of which occur in multiple animal species, including humans and wildlife (OIE 2019). OIE member countries are obligated to report on the status of these diseases and infections (presence or absence) every six months or immediately if the disease represents an exceptional event for that country. Most countries harmonise their list of notifiable conditions with the OIE list. They are assisted by tools provided by the OIE which are used to evaluate the performance of veterinary services and strengthen national veterinary services, ensuring high quality of domestic surveillance activities and programs (OIE PVS Tool, http://bit.ly/2zKJJry). Data provided by member countries are made publicly available through OIE’s World Animal Health Information System (WAHIS, http://bit.ly/2QmB49a) in an effort to improve transparency of the animal health situation in each country for trade purposes.
With trade a key driver of animal health surveillance, governments typically focus on economically important species, namely terrestrial livestock and, increasingly, aquatic animals. Passive surveillance methods and surveys to demonstrate freedom from disease comprise the bulk of surveillance activities for these species. By contrast, wild and companion animals usually fall outside of standard government structures for surveillance and different approaches are used to monitor disease in these animals.
Disease surveillance and wild animals
Compared to disease surveillance in livestock, wildlife surveillance is less regulated and responsibilities regarding wildlife health are often poorly defined. As a result, governance at the national level often falls into the jurisdiction of multiple agencies with nomination of a Focal Point for Wildlife (Table 8.1). Until recently there was no international agreement on what constituted priority diseases for surveillance in wild animals, although, as mentioned above, many of the OIE-listed diseases affect multiple species, including wildlife. The OIE Working Group on Wildlife has further identified ‘non-listed’ diseases which countries should target for surveillance based on their importance to wildlife conservation and potential to serve as an early warning for threats to livestock and/or public health. Countries can voluntarily report these non-listed diseases affecting wild animals, through OIE’s WAHIS-Wild interface (http://bit.ly/2QgBNbK). Non-listed disease data collected by OIE do not influence trade policy. Since 2016, countries reporting OIE-listed diseases occurring in wild animals do so through the regular WAHIS system.
A number of factors and issues inherent in the nature of wild animals complicate surveillance in this sector (Morner et al. 2002; Stallknecht 2007). Signs and symptoms in wildlife often differ from those in domestic animals. Many infections, being subclinical, remain undetected unless unusual events such as mass die-offs trigger investigation. Wildlife surveillance systems usually comprise opportunistic sampling to determine cause of death or disease rather than systematic sampling for specific pathogens. Because necropsy and histologic examinations are often unable to establish a cause of death or disease, laboratory diagnostic tests are required. The majority of laboratory tests, however, are only validated for humans, livestock and/or companion animals and their validity and accuracy on wildlife samples are frequently unknown. Wildlife-specific tests constitute a niche market and their development is neither cost-efficient nor industry driven, resulting in a lack of readily available diagnostic tools.
Wildlife comprises a wide range of taxa and habitats including aerial, aquatic and terrestrial animals. Many species are well camouflaged, live in areas difficult to access and sparsely populated, or undertake migration movements and frequently cross administrative boundaries. Because free-ranging wildlife is not under constant human observation, information is scarce and denominator data is lacking for calculations such as incidence or prevalence, which makes assessing the impacts of events difficult. Several techniques for estimating size and density of wildlife populations exist, including: direct (aerial surveys, drive counts) or indirect observation (tracks, nests, burrows or excretions); use of hunter harvest records; and capture–recapture methods. A collaborative project in Europe on harmonised approaches to monitoring wildlife population health and ecology and abundance (APHAEA, http://bit.ly/2zJQwBS) has recognised the need for accurate population estimates and has compiled fact cards for key species.
Due to the specific characteristics mentioned above a co-ordinated but decentralised data collection approach supported by citizen science is well suited for wildlife surveillance to encourage and ensure observation and reporting of unusual events by as many people as possible (Lawson, Petrovan and Cunningham 2015). These approaches still have to contend with issues of reliability, standardisation and harmonisation of reports and the fear that reports of wildlife diseases may impact on livestock trade or other activities involving wildlife (hunting, tourism). In short, the management of wildlife diseases is complex. The typical livestock disease control measures (e.g. stamping out or vaccination) might not apply to wildlife, and disease detection may be counter-productive to the conservation of endangered species. Despite progress in recent years many hurdles remain including sustainable funding for the ongoing collection and analysis of wildlife data at a country level. Readers interested in learning about the approaches to wildlife surveillance are referred to OIE’s training manual on surveillance and international reporting of diseases in wild animals (OIE 2015).
Surveillance in companion animals
Disease surveillance in companion animals such as dogs and cats is lacking in most countries, both high- and low-income. A number of OIE-listed diseases and infections occur in companion animals (e.g. rabies, Q fever/coxiellosis, hydatids) but, apart from rabies, few countries have surveillance systems in place to collect data and report these diseases in companion animal species (Day et al. 2012). Diseases and infections such as canine distemper virus and feline immunodeficiency virus while having a major impact on companion animals do not have implications for livestock and/or public health and so are not typically notifiable by law.
Some countries have surveillance platforms for collecting data on selected diseases (Glickman et al. 2006; Hennenfent et al. 2017; Ward and Kelman 2011). These systems rely on veterinarians entering case information using online platforms funded by the private sector or short-term government grants; at least one has been discontinued. Because these systems do not connect to any government agency responsible for companion animal health, it is unclear whether collation and analysis of information is undertaken such that a co-ordinated response to an outbreak could be mounted. Any decision to act on the information gathered through these surveillance systems sits with the end-user: the veterinary clinic inputting the data.
Challenges and opportunities in One Health surveillance
From the above discussion it is clear that, although both human and animal health sectors have similar objectives and approaches to surveillance, the motivations and reporting lines differ markedly between sectors. In addition, surveillance capacity across sectors and geographic regions differs considerably. Whereas public health and livestock surveillance is comparatively strong in high-income countries, these functions are fairly weak in low-income countries and wildlife and companion animal surveillance is virtually non-existent in most countries, both low- and high-income (Institute of Medicine [IOM] and National Research Council [NRC] 2009). To improve One Health outcomes, we need to strengthen surveillance capacity within sectors as well as expand opportunities for collaboration and integration across sectors. A number of challenges exist and are discussed in this section.
Structural and organisational barriers
In most countries and states disease surveillance of humans and animals is the responsibility of multiple organisations. This governance fragmentation affects all levels of infectious disease surveillance and response: separate laboratories detecting the same pathogens; distinct units investigating and following-up cases; different agencies collating data; notifications to separate intergovernmental bodies. The divide has practical ramifications for One Health activities such as lack of harmonisation of laboratory methods, weak communication channels across sectors, and legal and technical hurdles to real-time sharing of information. Mechanisms for funding cross-sectoral initiatives are rarely in place. Gaps in information sharing between the public and private sectors are also evident (IOM and NRC 2009).
Some countries have managed these barriers by establishing multi-sectoral task forces to facilitate joint planning and budgeting, particularly during outbreak periods (Morse 2014; Stark et al. 2015). More often cross-sectoral collaboration occurs via informal networks of trusted peers (Stark et al. 2015). In trying to formalise these networks, some countries have found it helpful to conduct a network analysis to better understand the stakeholder organisations and nature of the collaboration between sectors involved in One Health activities (Kimani et al. 2016; Sorrell et al. 2015). The increasing incidence of emerging diseases has prompted a number of regional disease surveillance networks to adopt One Health approaches, such as the Mekong Basin Disease Surveillance and East African Integrated Disease Surveillance Network (EAIDSNet) (Bond et al. 2013). Networks of networks have also formed, such as the non-governmental organisation, Connecting Organizations for Regional Disease Surveillance (CORDS), with the objective of improving coordination between human, animal and environmental sectors (Gresham et al. 2013). Addressing these existing structural barriers to One Health remains an area of intense and ongoing activity.
Differing disease priorities
Related to the above structural barriers are the different mandates and priorities of agencies involved in surveillance which provide limited opportunities for direct overlap. This is true even in the context of zoonoses, which might be expected to be an area of common interest to both human and animal health sectors. To illustrate this barrier, we compared notification lists for three countries with similar surveillance capacity (Australia, the United Kingdom and the United States of America) (Table 8.2). When there was an overlapping interest it was often confined to uncommon or rare diseases which limits occasions for combining datasets to enhance epidemiological knowledge. Zoonotic diseases in animal populations such as Q fever/coxiellosis, leptospirosis and chlamydiosis/ornithosis are not necessarily notifiable by veterinarians, yet are of public health importance. This reflects the trade-emphasis of animal health surveillance and the fact that many zoonotic infections do not cause illness in animals and so are not considered a major animal health issue.
|
Disease |
Australia1 |
UK |
USA | |||
|---|---|---|---|---|---|---|
|
|
Animal |
Human |
Animal |
Human |
Animal |
Human |
|
Australian bat lyssavirus |
X |
X |
|
|
|
|
|
Anthrax |
X |
X |
X |
X |
X |
X |
|
Babesiosis |
X |
|
|
|
X |
X |
|
Brucellosis |
X |
X |
X |
X |
X |
X |
|
Chlamydiosis/Ornithosis |
X |
X |
|
X |
X |
X |
|
Echinococcosis/Hydatids |
|
X |
|
|
X |
|
|
Hendra virus infection |
X |
X |
|
|
|
|
|
Influenza |
X |
X |
X |
X |
X |
X |
|
Japanese encephalitis |
X |
X |
|
|
X |
|
|
Leptospirosis |
|
X |
|
X |
X |
X |
|
Nipah virus infection |
X |
|
|
|
X |
|
|
Plague (Yersinia pestis) |
|
X |
|
X |
|
X |
|
Q fever/Coxiellosis |
|
X |
|
X |
X |
X |
|
Rabies |
X |
X |
X |
X |
X |
X |
|
Rift Valley fever |
X |
|
X |
X |
X |
|
|
Trichinellosis |
X |
|
|
|
X |
X |
|
Tularaemia |
X |
X |
|
X |
X |
X |
|
West Nile virus infection |
X |
X |
X |
X |
X |
X |
|
1 Notification of diseases varies between states of Australia. | ||||||
Table 8.2. A comparison of animal and human notifiable diseases for three selected countries of similar surveillance capacity. ‘X’ denotes those diseases that are notifiable in animals and humans.
Notwithstanding differing agency priorities and data requirements there will be many occasions when collating information is mutually beneficial even if additional information is required. It may be constructive for agencies to jointly agree on those diseases deserving of enhanced, cross-sectoral surveillance and response. This can be challenging in the context of data scarcity, particularly on the animal health side. To address this challenge the US Centers for Disease Control and Prevention (CDC) has developed a decision support tool to facilitate transparent priority setting by stakeholders involved in zoonotic disease surveillance, response and research (Rist, Arriola and Rubin 2014). Importantly, the tool is suitable for use in countries where surveillance data may be lacking.
Information sharing and data linkage
Surveillance data germane to One Health is often stored in different databases and from different organisations, and there are legal and technical barriers to information sharing and data linkage. One initial obstacle to data sharing across agencies is confidentiality of information, although in our experience this is often overcome at the network level when trust has been achieved. On the other hand, while sharing of data is operationally possible, accepted and desired, barriers may still exist at the policy level that prevent sharing of data (Stark et al. 2015). Differing spatial resolutions and lack of timeliness are other barriers to real-time data linkage (Wendt, Kreienbrock and Campe 2016).
Sharing information between private and public sectors is particularly challenging in the animal health sector as disclosure of information can impact the financial standing and market access of commercial entities (Stark et al. 2015). For some pathogens, these challenges are overcome by supporting anonymous sharing of isolates by animal industries for purposes of testing and sequencing. This approach has been used for influenza virus surveillance in swine in the United States (Animal and Plant Health Inspection Service [APHIS] n.d.). This approach impedes traceability and therefore would not be suitable for all pathogens.
Sharing pathogen databases also highlights the lack of harmonisation of molecular typing methods within and across sectors. Variable uptake of new diagnostic technologies and typing practices results in un-matched data collections which impede the ability to link data (Wendt, Kreienbrock and Campe 2015). The use of the WGS is advancing rapidly in public health and may replace other typing systems; however, introduction of this technology will be gradual and inevitably multiple typing systems will exist until concord is reached. WGS is yet to be incorporated into routine veterinary surveillance, leaving major gaps in our understanding of the microbial diversity of domestic and wild animals.
Human resources
Effective surveillance systems require people trained in the principles and practical aspects of monitoring diseases. Knowledge of applied epidemiology and skills in data analysis and communication help in surveillance activities (M’ikanatha et al. 2013). Field epidemiology training programs (FETPs) – modelled after the US CDC’s Epidemic Intelligence Service – have been the cornerstone of applied epidemiology training globally. In recent years, a number of FETPs have specifically catered for veterinarians with the aim of strengthening field veterinary services (Iamsirithaworn et al. 2014). Some programs deliberately engage both human and animal health professionals (Becker et al. 2012; Monday et al. 2011). Combined training in One Health surveillance is an excellent foundation, bringing medical practitioners, veterinarians, and environmental health professionals from different governmental sectors together with the common purpose of disease control and prevention (Monday et al. 2011).
One Health approaches to surveillance
The different drivers and motivations underpinning surveillance in humans and other animals means that certain approaches will always be needed to meet the individual requirements of each sector. Where overlap exists, efforts to integrate information and/or undertake joint data collection could lead to improved prediction of the risks to human and animal health, earlier detection of outbreaks and faster responses in both sectors. Such approaches have been used for some diseases, such as Rift Valley fever (see Box 8.3). Following is a discussion of areas which would benefit most from One Health surveillance.
Zoonotic diseases
Surveillance systems using a One Health approach to monitor zoonoses are generally divided into: 1) those aiming to integrate data collected through existing surveillance systems; and 2) those in which data are collected for an identified purpose using a coordinated approach. Wendt, Kreienbrock and Campe (2015) recently conducted a review of systems for integrating human and animal data on zoonoses. Of the 20 systems identified, most were established in the last decade and focused on early detection of new and emerging threats rather than endemic zoonoses. This is consistent with recent global interest and investment in the former, following major outbreaks of SARS and H5N1 influenza around 2003. These outbreaks contributed to the realisation that animals, particularly wildlife, were the sources of many recently emerged infections in humans (Jones et al. 2008) leading to proposals to redirect investment in zoonotic disease surveillance upstream, towards the wildlife source (Heymann and Dixon 2013; Karesh et al. 2012) (Figure 8.2).
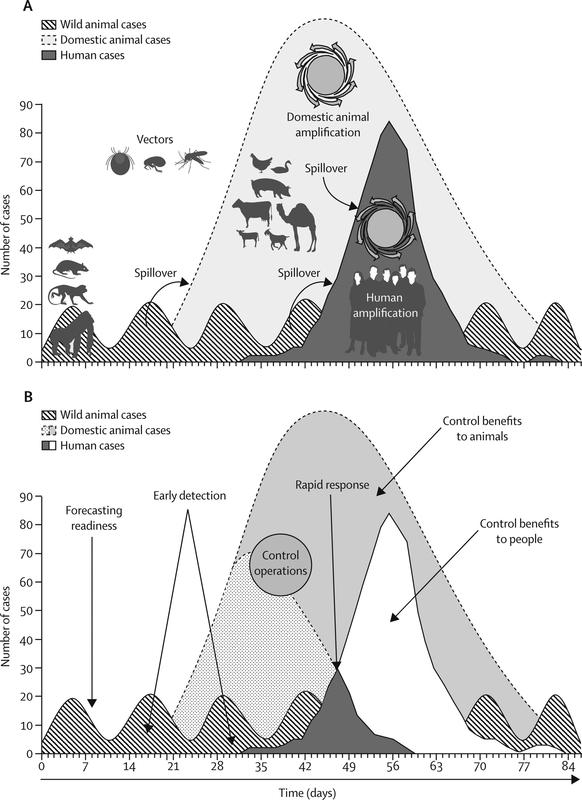
Figure 8.2 Upstream detection of zoonoses. (A) Transmission of infection and amplification in people (dark grey) occurs after a pathogen from wild animals (diagonal lines) moves into livestock to cause an outbreak (light grey) that amplifies the capacity for pathogen transmission to people. (B) Early detection and control efforts reduce disease incidence in people (white) and animals (dotted grey). Spillover arrows show cross-species transmission. Used with permission, Karesh et al. (2012).
At the global level, zoonotic disease surveillance systems that collate existing data include: Global Early Warning System for Major Animal Diseases, including Zoonoses (GLEWS; http://bit.ly/2zP08uZ), a joint initiative of WHO/FAO/OIE which aims to collate information on disease events gathered by each organisation; Global Public Health Intelligence Network (GPHIN), which is a web-based platform tracking disease outbreaks in humans, animals and plants reported through websites, news wires and other internet-based outlets; Global Outbreak Alert and Response Network (GOARN), which is a network of institutions and organisations providing technical support for rapid identification, confirmation and response to international outbreaks; and Program for Monitoring Emerging Diseases (ProMED-mail; www.promedmail.org) which collates information on human, animal and plant diseases retrieved from official and informal (media) reports as well as local observers.
A number of small-scale initiatives for joint data collection are reported in the literature, in particular for neglected zoonoses, such as brucellosis (reviewed by Zinsstag et al. 2015), and for disease surveillance in remote or Indigenous populations (Schurer et al. 2013; Schurer et al. 2014). Recent financial investments by international donors have resulted in the establishment of a number of large surveillance initiatives targeting high-risk populations in emerging disease ‘hotspots’. For example, the Vietnam Initiative on Zoonotic Infections (VIZIONS) – funded by the Wellcome Trust – is a countrywide project that encompasses hospital surveillance of four key syndromes associated with zoonotic infections as well as active surveillance of a high-risk cohort comprising farming households and others who work with domestic and wild animals (Rabaa et al. 2015). During human disease events VIZIONS conducts sampling of animals in the household environment and subjects both human and animal samples to microbiological testing, including genomic and phylogenomic analysis, to detect known and novel viruses. Participant observation and in-depth interviews are also conducted with individuals exposed to wildlife, to assess the contextual and behavioural risk factors in these groups. Analogous projects have been initiated in Mozambique (Gudo et al. 2016) and Kenya (Thumbi et al. 2015).
Similarly, the PREDICT project – which is part of the United States Agency for International Development’s (USAID) Emerging Pandemic Threats program – operates across more than 20 countries in central Africa, South and South-East Asia and parts of Latin America (Kelly et al. 2016). PREDICT works through a consortium of global and in-country partners to enhance surveillance of potential zoonotic viruses using ‘risk-based approaches’. Sampling of wildlife taxa that have been found to harbour zoonotic viruses (particularly those that have spilled over into humans including non-human primates, bats and rodents; Levinson et al. 2013) is prioritised. Laboratory protocols focus on detection of known and novel viruses belonging to viral families that have previously been associated with emerging disease outbreaks in humans. Central to PREDICT’s mission is development of in-country human and diagnostic capacity for wildlife disease surveillance. Training in safe and humane methods for wildlife sampling, including development of novel, non-invasive methods, is a key component of the project (Smiley Evans et al. 2015).
Whether projects such as VIZIONS and PREDICT are sustainable over the longer term is uncertain, but they illustrate the possibilities when adequate financial and political support is available. Through establishing networks of partners spanning human and animal health at national, regional and international levels, projects such as these can serve as a platform for scaling up approaches to zoonotic threats, including endemic diseases, in the future (Kelly et al. 2016).
Food-borne diseases
An important area of commonality between human and animal health is food production and a combined approach to the mitigation of food-borne disease. This includes the primary responsibility of animal feed manufacturers and farmers to ensure food safety through healthy animal production, as well as the responsibility of processors, retailers and consumers in preventing contamination further downstream. Food animals often harbour microorganisms in their normal flora that are pathogenic to humans. Poultry, for example, are often infected with Campylobacter and Salmonella resulting in frequent contamination of chicken meat and eggs. Fruit and vegetables may be contaminated with human and animal pathogens at any point during the production chain, including during the washing, packing and transportation processes. The source of outbreaks is often unknown which limits the implementation of effective whole-of-chain programs to prevent human infection (Galanis et al. 2012).
Surveillance for food-borne diseases is a core function of public health agencies and one likely to benefit from an integrated One Health approach. Use of molecular typing methods, such as pulse-field gel electrophoresis (PFGE) and multiple-locus variable number tandem repeat analysis (MLVA), enables rapid detection of clusters by identifying related isolates and establishing links between cases and potential sources. Owing to its improved discriminatory power, WGS is increasingly used in routine applications to further aid in source identification and source attribution (Vongkamjan and Wiedmann 2015). Data generated through these methods are shared via laboratory networks, such as US CDC PulseNet (http://bit.ly/2BUqNYS) and PulseNet International (http://bit.ly/2UjnotI) to better understand food-borne disease trends. To date, these techniques have primarily been used for assessing human samples and food items implicated in outbreaks. There has been limited application of these methods to food animals, feed and associated production environments.
A whole-of-chain approach to surveillance for food-borne pathogens is rare although there are some exceptions. In Canada, there have been efforts to integrate Salmonella data collected through several surveillance programs including data generated from (human and animal) diagnostic samples as well as active sampling of farms and feed ingredients, abattoirs, retail meats and surface water (Parmley et al. 2013). Similar approaches are being adopted in lower-income countries, such as Mexico (Zaidi et al. 2008) and Brazil (Dias et al. 2016). Enhanced molecular evaluation of potential reservoirs and vehicles – at all stages of the food chain – is contributing to improved source attribution and enabling the development of evidence-based policies. Farm-level interventions such as poultry vaccination and pre-chilling of chicken carcasses in the abattoir setting are some examples. Ongoing, integrated surveillance can be used to understand the impact of these interventions on the human burden of food-borne disease.
Antimicrobial resistance
Antimicrobial resistance (AMR) is a major threat to global health security (WHO 2014). Although often portrayed as a human health issue, AMR can be broadly framed as an ecological problem – with impacts on human, animal (domestic and wild) and plant health, as well as food hygiene and the environment (Butaye et al. 2014; Queenan, Hasler and Rushton 2016; Radhouani et al. 2014). In the context of AMR, surveillance is critical for understanding the extent of the problem, as well as evaluating the impact of interventions, such as revised infection control guidelines in (medical and veterinary) hospitals or policies regarding antimicrobial usage. Furthermore, advances in molecular characterisation of organisms have allowed a greater appreciation of the epidemiology of AMR and the movement of organisms and genes within and between environments and populations. In addition to data on the occurrence of AMR, information on antibiotic consumption is also a critical element of an AMR surveillance system, since antibiotic usage is a major cause of the problem (Queenan, Hasler and Rushton 2016).
Except for mandatory notifiable data collection (restricted to a limited range of conditions), it is difficult to obtain fully representative population-based antibiotic susceptibility data. Private laboratories in the health sector often refuse to share, citing confidentiality requirements. This results in data collections biased towards acute hospital cases; data is lacking from the general community. The limited collation of data between medical and veterinary laboratories further constrains our understanding.
The recent WHO Global Report on Surveillance noted major shortcomings in global capacity for AMR surveillance; specifically the lack of consensus on methodology, limited data sharing and poor co-ordination across geographic regions (WHO 2014). While a number of national and regional surveillance networks for AMR in human health have been established in recent decades, most focus on high-income countries, relate to specific pathogens, and/or data are not systematically obtained or geographically representative (Dar et al. 2016). The lack of internationally agreed standards for data collection and reporting on AMR in human health limits comparability of the data across countries and regions. In 2012 international standards on harmonisation of national AMR surveillance were adopted by OIE members (OIE 2012); however, at the time most countries lacked an official system for gathering data on antimicrobial usage in animals (Nisi et al. 2013). Lack of (medical and veterinary) microbiology diagnostic facilities remains a major constraint to AMR surveillance in low- and middle-income countries (Dar et al. 2016), and the same is true for the veterinary sector in many higher-income countries.
With these shortcomings in mind, the World Health Assembly adopted the Global Action Plan on AMR, in 2015 – a specific objective of which is to ‘strengthen the knowledge and evidence-base through surveillance and research’. Subsequently, WHO/OIE/FAO launched a number of initiatives to support WHO member countries in developing and implementing surveillance systems for AMR and antibiotics usage in humans and animals. WHO launched the Global Antimicrobial Resistance Surveillance System (GLASS) to support standardised approaches to the collection, analysis and sharing of data at the global level. Likewise, as part of the OIE Strategy on AMR and the Prudent Use of Antimicrobials, OIE is developing a global database on the use of antibiotics in livestock and companion animals (OIE 2016). These new initiatives – which aim to strengthen sectoral approaches to AMR surveillance – were enhanced when WHO/OIE/FAO called for improved integration of surveillance for food-borne pathogens, with coordinated sampling and testing of bacteria from livestock, foods, the environment as well as clinically ill humans (Acar and Moulin 2013) (see Figure 8.3).
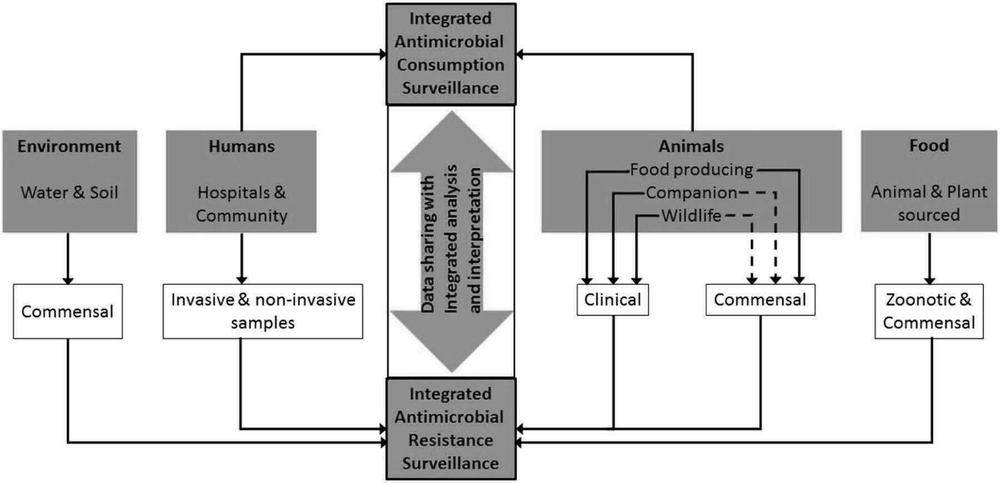
Figure 8.3 Framework for integrated surveillance on antimicrobial resistance and antimicrobial consumption. Used with permission, Queenan, Hasler and Rushton (2016).
A number of high-income countries have already made significant progress toward establishing integrated surveillance systems for AMR. The Danish Integrated Antimicrobial Resistance Monitoring and Research Program (DANMAP) was established in 1995 and collects and analyses data on antibiotic use and AMR in select indicator, zoonotic and pathogenic bacteria. Isolates come from a variety of sources, including: healthy animals at slaughter; sick animals subjected to diagnostic investigation; foods retrieved from wholesale and retail outlets as part of routine inspections; healthy humans; and humans subjected to diagnostic evaluation following contact with the healthcare system (Hammerum et al. 2007). Evidence generated through this integrated surveillance system was instrumental in supporting policy changes around the use of antibiotics in livestock, both in Denmark and Europe (Hammerum et al. 2007). Similar integrated surveillance systems were established in other high-income countries, and include the Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) and the National Antimicrobial Resistance Monitoring System (NARMS) in the United States. Interested readers are referred to Acar and Moulin (2013) for a more comprehensive listing of such systems. More recently, lower-income countries, such as Mexico (Zaidi et al. 2008) and Colombia (Donado-Godoy et al. 2015) have established similar programs.
While integrated approaches appear to be the future of AMR surveillance, Queenan, Hasler and Rushton (2016) note that none of the current systems are fully integrated because wild and companion animals and the environment are not under surveillance. Resistant microbial populations have been detected in a variety of environmental samples, including hospital and farm effluent, sewage, and wastewater, indicating that AMR can spread through the environment (see review by Singer et al. 2016). Wild animals – rarely directly exposed to antimicrobial agents – have been found to acquire antimicrobial-resistant bacteria (Radhouani et al. 2014). Concerns about the potential for wild animals, particularly migrating birds, to disseminate resistance genes throughout the environment have been raised (see recent review by Arnold, Williams and Bennett 2016). It has been suggested that sentinel surveillance comprising sampling of high-risk environments would be an appropriate addition to enhance monitoring of AMR (Dar et al. 2016). While GLASS encourages participating countries to collect data on human bacterial pathogens, there are plans to expand data collection to the food chain and environment (WHO 2015).
Vector-borne diseases and other environmental hazards
Earlier we introduced the concept of sentinel surveillance, which involves monitoring of health events through sentinel sites, providers or vectors/animals. With regard to the latter, sentinel surveillance has been applied to monitor circulation of various human pathogenic viruses and bacteria (see Table 8.3). These pathogens share similar features, notably a highly sensitive animal host and an environmental component to transmission. The latter may include a non-vertebrate vector or environmentally stable form such as spores. Given the sensitivity of such diseases to climate-related parameters, there has been substantial growth in methods linking meteorological data to disease data, to enhance surveillance and perform better risk analysis and prediction. For example, using data on changes in climate to predict the human health risk from Ross River virus is now an annual surveillance method deployed in Australia (Woodruff et al. 2006). Climate modelling combined with animal and human health surveillance can be used to better understand and respond to changing risk and is becoming increasingly important with global climate change. Modelling of changes to weather patterns in Zimbabwe, for example, combined with surveillance for parasitic worms in humans and snails, has shown that schistosomal risk will be reduced in some existing areas and increased in previously unaffected areas (Pedersen et al. 2014).
|
Pathogen |
Disease |
Vector |
Animal sentinel(s) |
|---|---|---|---|
|
Bacillus anthracis |
Anthrax |
NA |
Cattle and sheep |
|
Borrelia burgdorferi |
Lyme disease |
Ticks |
White-tailed deer |
|
Clostridium botulinum |
Botulism |
NA |
Waterfowl |
|
Leishmania sp. |
Leishmaniasis |
Sandflies |
Domestic dogs |
|
Murray Valley encephalitis virus |
Murray Valley encephalitis |
Mosquitoes |
Chickens |
|
West Nile virus |
West Nile fever |
Mosquitoes |
Crows |
|
Yellow fever virus |
Yellow fever |
Mosquitoes |
Non-human primates |
|
Yersinia pestis |
Plague |
Fleas |
Domestic cats |
Table 8.3. Examples of human pathogens that are monitored through surveillance of an animal sentinel. NA, not applicable.
So far, we have only considered infectious hazards with strong links to the environment. Any discussion of environmental hazards must also consider non-infectious threats to human and animal health. According to Thacker et al. (1996), surveillance for such hazards typically encompasses three levels of investigation:
- hazard surveillance, which establishes the presence of a particular hazard(s) in the environment (e.g. air pollution)
- exposure surveillance, which establishes the extent of exposure to a particular toxin/chemical agent in the population (e.g. lead poisoning)
- outcome surveillance, which monitors the frequency of adverse effects following exposure (e.g. birth defects, cancer).
In theory, all levels of investigation lend themselves to One Health approaches. In hazard surveillance, aquatic and terrestrial animals, including humans, are all potentially impacted by pollutants present in air, water and soil, and efforts to monitor and mitigate environmental release would likely benefit all species. Similarly, exposure and outcome surveillance could include monitoring chemical hazards and associated outcomes in aquatic and terrestrial animals, including humans, for the purposes of inferring dangers present to other species. Perhaps the best-known example of this is the use of canaries to detect toxic levels of carbon monoxide and methane in coalmines.
Mercury is another example where One Health surveillance may provide broad value. Mercury in the environment can have serious adverse effects across all biological systems. A striking illustration of this followed the outbreak of Minimata disease in Japan in the 1950s, when a handful of cases of a mysterious neurological illness started appearing in residents of Minimata (Harada 1995). Investigations revealed that city residents had observed strange behaviours in animals: cats were developing convulsions and dying (so-called dancing cat disease), birds were falling from the sky, and fish in Minimata Bay were floating belly-up on the surface. Many of the human patients resided on the shores of Minimata Bay and consumed fish and shellfish from the bay. Subsequent hazard surveillance confirmed high levels of organic mercury in fish, shellfish and sludge of Minimata Bay. Experimental studies in cats confirmed mercury toxicity. Regrettably, the investigation findings were not immediately accepted, allowing contamination to continue. According to official figures, around 2,252 people developed Minimata disease (1,043 died), although many thousands went undiagnosed (Harada 1995).
Lead is another case where One Health surveillance using animal sentinels has proved successful. In the town of Esperance, Western Australia, in 2006, authorities were alerted to a potential environmental disaster following a mass mortality event that affected 10,000 song birds. The deaths were attributed to lead poisoning and isotopic investigations confirmed the same signature in samples collected from birds, humans and the environment, including drinking water and soil (Gulson et al. 2009). The lead carbonate came from a nearby mine, which transported the ore through the town to the port. The sentinel event in animals triggered an investigation that showed lead dust was escaping at the port. Exposure surveillance confirmed that humans in the town had elevated blood lead levels, but very few cases of lead poisoning eventuated because of swift intervention.
Both the Minimata and Esperance incidents illustrate the potential for animals to serve as sentinels for environmental threats to human health. Readers with a particular interest in environmental health applications are referred to the Canary Database, which compiles research articles on the use of animals as sentinels of human health hazards (canarydatabase.org). It would be remiss of us not to mention that humans may also serve as sentinels for animal health events, and often do, given more comprehensive health assessment. This is how many recent emerging diseases – such as SARS – have come to be recognised.
Conclusion
This chapter has described a range of surveillance options and challenges to monitoring disease at the human–animal–environment interface. The principal objective is to design systems that meet the individual requirements of each sector, while providing a more comprehensive picture of the health risks to all populations. Without integration and an overarching appreciation of universal objectives, surveillance systems will only ever be as good as the sum of each separate component. One Health surveillance can build new understandings, give new ways of seeing issues and develop new solutions. It is the intersecting places, issues and ideas of One Health where new advances are possible.
One Health surveillance needs champions committed to harnessing the energy and building trust across sectors. Trust is more often built by deeds than words. We conclude with a challenge to readers to seek new collaborations, merge skills, share knowledge, broaden access and open up opportunities that are unattainable when we work within silos. Many One Health surveillance approaches can be developed, trialled and reported. Over time and through evaluation and sharing of learning, One Health surveillance will move from a few examples to effective and ongoing systems that benefit the whole planet.
Recommendations
These recommendations are directed at local, regional and national levels as One Health issues are not bound by borders or jurisdictions – all levels are interrelated:
- Actively develop opportunities to discuss and build One Health surveillance collaborations. In the first instance, this may occur through informal collaboration with the aim of progressing towards more formal agreements with specific terms of reference.
- Develop support at the local level for One Health surveillance through collection of data on environment, human and animal health parameters. Support may include incentives that promote inclusion and communication between diverse stakeholders.
- Develop, trial and evaluate new ways of combining surveillance data at regional and national levels to build new understandings of health risk and connectedness.
- Collaboratively and jointly report and present surveillance data in each sector.
- Advocate for political, legal and ethical frameworks for sharing surveillance data and building a One Health surveillance network.
- Engage in research collaborations using inter-agency data access and the combined skills of professionals to gather information unobtainable to individual organisations.
Works cited
Acar, J.F., and G. Moulin (2013). Integrating animal health surveillance and food safety: the issue of antimicrobial resistance. Rev Sci Tech 32: 383–92.
Allepuz, A., de Balogh, K., Aguanno, R., Heilmann, M., Beltran-Alcrudo, D. 2017. Review of Participatory Epidemiology Practices in Animal Health (1980-2015) and Future Practice Directions. PLOS ONE 12(1): e0169198. doi: 10.1371/journal.pone.0169198.
Animal and Plant Health Inspection Service. Influenza virus surveillance in swine: program overview for veterinarians. http://bit.ly/2AxYOft.
Arnold, K.E., N.J. Williams, and M. Bennett (2016). Disperse abroad in the land: the role of wildlife in the dissemination of antimicrobial resistance. Biology Letters 12(8): 2016.0137.
Becker, K.M., et al. (2012). Field epidemiology and laboratory training programs in West Africa as a model for sustainable partnerships in animal and human health. Journal of the American Veterinary Medical Association 241(5): 572–579.
Bird, B. H., et al. (2008). Multiple virus lineages sharing recent common ancestry were associated with a large Rift Valley fever outbreak among livestock in Kenya during 2006-2007. Journal of Virology 82(22): 11152–11166.
Bond, K.C., S.B. Macfarlane, C. Burke, K. Nguchusak, and S. Wibulpolprasert (2013). The evolution and expansion of regional disease surveillance networks and their role in mitigating the threat of infectious disease outbreaks. Emerging Health Threats Journal 6: 10.3402/ehtj.v6i0.19913.
Bruckner, G.S., et al. (2010). Handbook on import risk analysis for animals and animal products. Volume 1: introduction and qualitative risk analysis. Paris: World Organisation for Animal Health.
Butaye, P., E. Van Duijkeren, J.F. Prescott, and S. Schwarz (2014). Antimicrobial resistance in bacteria from animals and the environment. Veterinary Microbiology 171(3–4): 269–272.
Catley, A., R. G. Alders and J. L. Wood (2012). Participatory epidemiology: approaches, methods, experiences. Veterinary Journal 191(2): 151–160.
Centers for Disease Control and Prevention (2007). Rift Valley fever outbreak - Kenya, November 2006-January 2007. MMWR: Morbidity and Mortality Weekly Report 56(4): 73–76.
Dar, O.A., et al. (2016). Exploring the evidence base for national and regional policy interventions to combat resistance. Lancet 387: 285–95.
Day, M.J., et al. (2012). Surveillance of zoonotic infectious disease transmitted by small companion animals. Emerging Infectious Diseases 18(12). https://bit.ly/2HSvnvH.
Dias, M.R., et al. (2016). Molecular tracking of Salmonella spp. in chicken meat chain: from slaughterhouse reception to end cuts. Journal of Food Science and Technology 53(2): 1084–1091.
Donado-Godoy, P., et al. (2015). The establishment of the Colombian Integrated Program for Antimicrobial Resistance Surveillance (COIPARS): a pilot project on poultry farms, slaughterhouses and retail market. Zoonoses Public Health 62(Supplement 1): 58–69.
Dorea, F.C., J. Sanchez, and C.W. Revie (2011). Veterinary syndromic surveillance: current initiatives and potential for development. Preventive Veterinary Medicine 101(1–2): 1–17.
Galanis, E., J. Parmley, N. De With, and B.C. Survei (2012). Integrated surveillance of Salmonella along the food chain using existing data and resources in British Columbia, Canada. Food Research International 45(2): 795–801.
Glickman, L.T., et al. (2006). Purdue University–Banfield National Companion Animal Surveillance Program for emerging and zoonotic diseases. Vector Borne Zoonotic Dis 6: 14–23.
Gresham, L.S., M.S. Smolinski, R. Suphanchaimat, A.M. Kimball, and S. Wibulpoprasert (2013). Creating a global dialogue on infectious disease surveillance: connecting organizations for regional disease surveillance (CORDS). Emerging Health Threats Journal 6: 10.3402/ehtj.v6i0.19912.
Gudo, E.S., et al. (2016). Mozambique experience in implementing One Health surveillance as an innovative tool to understand the risk of spillover of emerging and zoonotic infections between wildlife and humans. International Journal of Infectious Diseases 45(Supplement 1): 468.
Gulson, B., et al. (2009). Windblown lead carbonate as the main source of lead in blood of children from a seaside community: an example of local birds as ‘canaries in the mine’. Environmental Health Perspectives 117(1): 148–154.
Hammerum, A.M., et al. (2007). Danish integrated antimicrobial resistance monitoring and research program. Emerging Infectious Diseases 13(11): 1632–1639.
Harada, M. (1995). Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Critical Reviews in Toxicology 25(1): 1–24.
Hennenfent, A., V. Delvento, J. Davies-Cole, and F. Johnson-Clarke (2017). Expanding veterinary biosurveillance in Washington, DC: the creation and utilization of an electronic-based online veterinary surveillance system. Preventive Veterinary Medicine 138: 70–78.
Henning, K.J. (2004). What is syndromic surveillance? MMWR: Morbidity and Mortality Weekly Report Supplement 53: 5–11.
Heymann, D.L., and M. Dixon (2013). The value of the One Health approach: shifting from emergency response to prevention of zoonotic disease threats at their source. Microbiology Spectrum 1(1): 10.1128/microbiolspec.OH-0011–2012.
Iamsirithaworn, S., K. Chanachai, and D. Castellan (2014). Field epidemiology and One Health: Thailand’s experience. In Confronting emerging zoonoses: the One Health paradigm, A. Yamada et al., eds. Japan: Springer. doi: 10.1007/978-4-431-55120-1.
Institute of Medicine and National Research Council (2009). Sustaining global surveillance and response to emerging zoonotic diseases. Washington, DC: The National Academies Press. https://doi.org/10.17226/12625.
International Society for Disease Surveillance (2017). One Health Surveillance Workgroup. https://bit.ly/2SBiV7w.
Jones, K.E., et al. (2008). Global trends in emerging infectious diseases. Nature 451: 990–3.
Jost, C. C., et al. (2010). Epidemiological assessment of the Rift Valley fever outbreak in Kenya and Tanzania in 2006 and 2007. American Journal of Tropical Medicine and Hygiene 83(Supplement 2): 65–72.
Karesh, W.B., et al. (2012). Ecology of zoonoses: natural and unnatural histories. Lancet 380: 1936–45.
Kelly, T.R., et al. (2016). One Health proof of concept: bringing a transdisciplinary approach to surveillance for zoonotic viruses at the human–wild animal interface. Preventive Veterinary Medicine 137(Part B): 112–118. doi: 10.1016/j.prevetmed.2016.11.023.
Kimani, T., M. Ngigi, E. Schelling, and T. Randolph (2016). One Health stakeholder and institutional analysis in Kenya. Infection Ecology & Epidemiology 6: 10.3402/iee.v6.31191
Kwong, J.C., N. Mccallum, V. Sintchenko, and B.P. Howden (2015). Whole genome sequencing in clinical and public health microbiology. Pathology 47: 199–210.
Lawson, B., S.O. Petrovan, and A. Cunningham (2015). Citizen science and wildlife disease surveillance. EcoHealth 12: 693–702.
Levinson, J., et al. (2013). Targeting surveillance for zoonotic virus discovery.Emerging Infectious Diseases 19(5): 743–747.
Lutomiah, J., D., et al. (2014). Blood meal analysis and virus detection in blood-fed mosquitoes collected during the 2006-2007 Rift Valley fever outbreak in Kenya. Vector Borne and Zoonotic Diseases 14(9): 656–664.
May, L., R.L. Katz, E. Test, and J. Baker (2011). Applications of syndromic surveillance in resource poor settings. World Medical and Health Policy 3(4): 1–29.
M’ikanatha, N.M., R. Lynfield, C.A. Van Beneden, and H. De Valk (2013). Infectious disease surveillance: a cornerstone for prevention and control In Infectious disease surveillance, 2nd edn., N.M. M’ikanatha, R. Lynfield, C.A. Van Beneden, and H. De Valk, eds. Sommerset, UK: Wiley-Blackwell.
Monday, B., et al. (2011). Paradigm shift: contribution of field epidemiology training in advancing the ‘One Health’ approach to strengthen disease surveillance and outbreak investigations in Africa. Pan African Medical Journal 10(Supplement 1): 13.
Morner, T., D.L. Obendorf, M. Artois, and M.H. Woodford (2002). Surveillance and monitoring of wildlife diseases. Revue Scientifique et Technique 21(1): 67–76.
Morse, S. (2014). Public health disease surveillance networks. Microbiology Spectrum 2(1). doi: 10.1128/microbiolspec.OH-0002-2012.
Murray, N., et al. (2010). Handbook on import risk analysis for animals and animal products. Volume 2: quantitative risk assessment. Paris: World Organisation for Animal Health.
Nisi, R., N. Brink, F. Diaz, and G. Moulin (2013). Antimicrobial use in animals: analysis of the OIE survey on monitoring of the quantities of antimicrobial agents used in animals. Paris: World Organisation for Animal Health. http://bit.ly/2EhgmAY.
Nuttall, I., K. Miyagishima, C. Roth, and S. De La Rocque (2014). The United Nations and One Health: the International Health Regulations (2005) and global health security. Revue Scientifique et Technique 33(2): 659–668.
Parmley, E.J., et al. (2013). A Canadian application of One Health: integration of Salmonella data from various Canadian surveillance programs (2005–2010). Foodborne Pathogens and Disease 10(9): 747–756.
Pedersen, U.B., et al. (2014). Modelling spatial distribution of snails transmitting parasitic worms with importance to human and animal health and analysis of distributional changes in relation to climate. Geospatial Health 8(2): 335–343.
Peeler, E.J., R.A. Reese, and M.A. Thrush (2015). Animal disease import risk analysis – a review of current methods and practice. Transboundary and Emerging Diseases 62(5): 480–490.
Queenan, K., B. Hasler, and J. Rushton (2016). A One Health approach to antimicrobial resistance surveillance: is there a business case for it? International Journal of Antimicrobial Agents 48(4): 422–427.
Rabaa, M.A., et al. (2015). The Vietnam Initiative on Zoonotic Infections (VIZIONS): a strategic approach to studying emerging zoonotic infectious diseases. EcoHealth 12: 726–35.
Radhouani, H., et al. (2014). Potential impact of antimicrobial resistance in wildlife, environment and human health. Frontiers in Microbiology 5: 23.
Rist, C.L., C.S. Arriola, and C. Rubin (2014). Prioritizing zoonoses: a proposed One Health tool for collaborative decision-making. PLOS One 9(10): e109986. doi: 10.1371/journal.pone.0109986.
Schurer, J.M., M. Ndao, H. Quewzance, S.A. Elmore, and E. Jenkins (2014). People, pets, and parasites: One Health surveillance in southeastern Saskatchewan. American Journal of Tropical Medicine and Hygiene 90(6): 1184–1190.
Schurer, J.M., et al. (2013). Parasitic zoonoses: One Health surveillance in northern Saskatchewan. PLOS Negl Trop Dis 7: e2141. doi: 10.1371/journal.pntd.0002141.
Singer, A.C., H. Shaw, V. Rhodes, and A. Hart (2016). Review of antimicrobial resistance in the environment and its relevance to environmental regulators. Frontiers in Microbiology 7: 1728.
Smiley Evans, T., et al. (2015). Optimization of a novel non-invasive oral sampling technique for zoonotic pathogen surveillance in nonhuman primates. PLoS Neglected Tropical Diseases 9(6): e0003813. doi: 10.1371/journal.pntd.0003813.
Sorrell, E.M., et al. (2015). Mapping of networks to detect priority zoonoses in Jordan. Frontiers in Public Health 3: 219.
Stallknecht, D. (2007). Impediments to wildlife disease surveillance, research, and diagnostics. Current Topics in Microbiology & Immunology 315: 445–61.
Stark, K.D., et al. (2015). One Health surveillance – more than a buzz word? Preventive Veterinary Medicine 120(1): 124–130.120.
Thacker, S.B., D.F. Stroup, R.G. Parrish, and H. Anderson (1996). Surveillance in environmental public health: issues, systems, and sources. American Journal of Public Health 86(5): 633–638.
Thumbi, S.M., et al. (2015). Linking human health and livestock health: a ‘One-Health’ platform for integrated analysis of human health, livestock health, and economic welfare in livestock dependent communities. PLOS One 10: e0120761. doi: 10.1371/journal.pone.0120761.
Vongkamjan, K., and M. Wiedmann (2015). Starting from the bench – prevention and control of foodborne and zoonotic diseases. Preventive Veterinary Medicine 118(2–3): 189–195.
Ward, M.P., and M. Kelman (2011). Companion animal disease surveillance: a new solution to an old problem? Spatial & Spatiotemporal Epidemiology 2(3): 147–157.
Wendt, A., L. Kreienbrock, and A. Campe (2015). Zoonotic disease surveillance – inventory of systems integrating human and animal disease information. Zoonoses Public Health 62(1): 61–74.
Wendt, A., L. Kreienbrock, and A. Campe (2016). Joint use of disparate data for the surveillance of zoonoses: a feasibility study for a One Health approach in Germany. Zoonoses Public Health 63(7): 503–14.
Woodruff, R.E., C.S. Guest, M.G. Garner, N. Becker, and M. Lindsay (2006). Early warning of Ross River virus epidemics: combining surveillance data on climate and mosquitoes. Epidemiology 17: 569–75.
World Health Organization (2005). International Health Regulations. Geneva: World Health Organization.
World Health Organization (2014). Antimicrobial resistance: global report on surveillance 2014. Geneva: World Health Organization.
World Health Organization (2015). Global Antimicrobial Resistance Surveillance System. Geneva: World Health Organization. http://bit.ly/2UqPF1J.
World Organisation for Animal Health (2012). Harmonisation of national antimicrobial resistance surveillance and monitoring programmes. Terrestrial animal health code. Paris: World Organisation for Animal Health.
World Organisation for Animal Health (2015). Training manual on surveillance and international reporting of diseases in wild animals. Paris: World Organisation for Animal Health.
World Organisation for Animal Health (2016). OIE strategy on antimicrobial resistance and the prudent use of antimicrobials. Paris: World Organisation for Animal Health. https://bit.ly/2GsR575.
World Organisation for Animal Health (2018). OIE-listed diseases, infections and infestations in force in 2018. Paris: World Organisation for Animal Health. http://bit.ly/2G5QmdH.
Zaidi, M.B., et al. (2008). Integrated food chain surveillance system for Salmonella spp. in Mexico. Emerging Infectious Diseases 14(3): 429–35. https://bit.ly/2t55s9O.
Zinsstag, J., et al. (2015). Brucellosis surveillance and control: a case for One Health. In One Health: the theory and practice of integrated health approaches, J. Zinsstag, E. Schelling, D. Waltner-Toews, M. Whittaker, and M. Tanner, eds. Wallingford, UK: CABI.
1 Rather than provide an estimate of disease prevalence, proof of freedom surveys provide evidence – with a degree of statistical confidence – that a particular disease is absent in animal populations in a given country or zone. Proof of freedom surveys are an essential tool in veterinary epidemiology. They provide evidence to trading partners that certain diseases are not likely to be present in animals from the exporting country, and thus the importing country can be assured that they are not going to introduce diseases as a result of trade between the two countries.
2 ‘Pathogen surveillance’ (as opposed to ‘disease surveillance’) is an important concept in animal health surveillance, since infection with a particular pathogen may not produce visible signs of disease in all species under surveillance (hence certain diseases and infections are notifiable). Occurrence of the pathogen is nonetheless important for understanding the potential for transmission between species, including to humans.