7
To screen or not to screen for prostate cancer?
We now turn to the “crunch” issue in this book, where we will try to provide men with the necessary information to assist them to make a truly informed choice about whether to get tested for prostate cancer.
What is meant by “screening” for a disease?
Screening is a process of identifying asymptomatic people who are at high risk of having or developing a particular disease or condition (often called the “target condition”). Screening has been described as “putting a population through a sieve” (see www.screening.nhs.uk/screening). Most people will pass through the sieve (screening test). These people are called low risk for the target condition; they receive a “normal” test result. Often they are asked to come back in a few years for another test. Some people get caught in the sieve. They are people who are at high (or at least higher) risk of having or developing the target condition. They will be offered follow-up tests to see if they really have the target condition or not. Usually the majority of them do not have the target condition; their experience is described as a “false positive”. However, some people really have the target condition (true positives) and they are offered treatment for it. The idea is that this early detection and early treatment of the target condition will produce better results than waiting for the disease or condition to cause symptoms and treating it then. 90
If it’s a good sieve (screening test), it lets through only low-risk people and catches all the high-risk people. Unfortunately none of our sieves are perfect – there are always some people who pass through the sieve who really are high risk and should have been caught (false negatives). Likewise not everyone who gets caught in the sieve actually has the disease or is at high risk of having it. Because our sieves are not perfect, the initial test (the sieve) never definitely tells whether the target condition is present or not. It just sorts people into low risk and high risk for the target condition. So an abnormal screening test always needs to be followed up with more investigations to confirm the initial suspicions.
One of the important things about screening is that the people who are screened (go into the sieve) are well. They do not already have the target condition, or any symptoms of it. If they do, they are not being screened, they are instead said to be having a “diagnostic” test to see what is the cause of their symptom. For example, a woman with no symptoms of breast cancer (such as a breast lump) may be screened by a mammogram. If her screening mammogram is abnormal, she will then be offered follow-up tests (which may include more mammograms, an ultrasound and/or a biopsy) to establish whether she has breast cancer or not. These follow-up tests may be called diagnostic tests, because they are done to establish a diagnosis, after the initial screening test has indicated she has something suspicious. A woman who already has a breast lump will also have a diagnostic mammogram to see if the lump is cancer or not. Even though it is the same test (a mammogram) this is not screening, this is diagnosis because she already has something suspicious (a lump). Her mammogram does not lead to the possibility of treating the cancer early, before it has caused any symptoms.
Another very important point about screening is that the screening test alone does not deliver any benefit. It is the package of the screening test plus early treatment that may deliver a health benefit. 91Just doing the test is only the first step of a screening program. Without an effective program which provides the follow-up test(s) and early, effective treatment for people who have the target condition, screening cannot possibly do any good. So it is best to think of screening as a screening program, rather than a screening test.
In Australia, blood taken from a heel prick of newborn babies is routinely screened to help identify over 20 metabolic conditions such as phenylketonuria (PKU – an enzyme deficiency disorder which if left untreated, can lead to mental retardation); homocystinuria (an inherited enzyme deficiency disease involving a build-up of the amino acid homocystine which can cause progressive mental retardation) and maple syrup urine disease (named after the presence of sweet-smelling urine in affected babies. If left untreated, infants suffer severe brain damage and eventually die.)
Screening of adults seeks to find evidence of chronic disease not yet causing symptoms and therefore not under medical care and may identify risk factors like high cholesterol and blood pressure, genetic pre-disposition or early evidence of disease – as is the case with colorectal, cervical and breast cancer screening.
Why do we screen for some diseases but not others?
In 1968, the World Health Organization published what would become a classic report in the history of modern medicine [103]. It set out a framework for deciding when it is worthwhile to screen. While it set out some very useful principles, it has been updated several times since as we have learnt more about screening and its pitfalls. For example in 2003, the National Screening Unit in New Zealand published a short set of criteria (see Table 10). Similar criteria have been developed and adopted to guide policy in the UK, Canada and the US. 92
Table 10: Criteria for assessing screening programs
The condition is a suitable candidate for screening.
There is a suitable test.
There is an effective and accessible treatment or intervention for the condition identified through early detection.
There is high quality evidence, ideally from randomized controlled trials, that a screening program is effective in reducing mortality and morbidity.
The potential benefit from the screening programme should outweigh the potential physical and psychological harm (caused by the test, diagnostic procedures and treatment).
The health care system will be capable of supporting all necessary elements of the screening pathway, including diagnosis, follow-up and program evaluation.
There is consideration of social and ethical issues.
There is consideration of cost-benefit issues.
Source: www.nsu.govt.nz/files/NSU/Screening_to_improve_health.pdf
These criteria are very important. Although it seems odd, a screening program which does not address these criteria may in fact do more harm than good. This is because screening is done to well people, so there is a real possibility of doing harm if the screening test or the following tests or the treatment for the target condition carry important risks. For this reason there is now broad agreement among expert groups that there must be “gold standard” evidence (mostly this means evidence from randomised controlled trials) that detecting disease early and treating it earlier than would otherwise have happened must reduce deaths or improve quality of life. In short, if treating a person’s disease at a very early time makes no positive difference to their life, why would you do it? You would be 93running the risk of giving people only more “disease-time” rather than more lifetime. The idea of adverse effects (or harmful effects) of screening is quite counterintuitive. But it is reasonable to think that all screening will do some harm.
Sometimes the harm is limited to the anxiety and inconvenience of undergoing the screening test. However it is vital to appreciate that screening is actually a “package deal” of early detection and early treatment if disease or pre-disease is found. If you don’t go on to treat what you find, there can be no benefit of screening. For example, what is the point of finding out your child has PKU if you are not going to do anything to modify their diet? Similarly is there really any point in finding out early you have breast or bowel cancer if you don’t intend to treat it? Therefore the adverse effects of treating screen-detected disease have to be considered as adverse effects of screening. And that’s very important in prostate cancer screening because as we saw earlier, adverse effects of follow-up tests and treatments for prostate cancer are common and can be severe.
The side effects of prostate cancer treatment are especially relevant when thinking about the “package deal” of prostate cancer screening because of the big reservoir of indolent (non-harmful) prostate cancer that we talked about before. This big reservoir of indolent prostate cancer in the population means that if we screen whole populations of men for the disease, we will find it in many of them. If we treat all those people, there is enormous potential to cause harmful effects in many men. This means it’s especially important in the case of prostate cancer screening to carefully consider whether the benefits of screening are likely to outweigh the harms. Soon we will take a detailed look at what we really know about the benefit of prostate cancer screening.
Health agencies around the world are increasingly recognising that many people want to be involved in decisions that affect their own 94health. Many people no longer want their doctor or their government to decide for them. Especially in “close-call” decisions where the beneficial effects and harmful effects may be finely balanced and in decisions where personal preferences may strongly influence what a person wishes to do, people want to have a say. Screening for prostate cancer is perhaps the best example available of such a “close call” and of what people studying this phenomenon call a “preference-sensitive decision” [104]. The US Preventive Services Taskforce assessment of the evidence for and against PSA screening concluded: “the current evidence is insufficient to assess the balance of benefits and harms of prostate cancer screening in men younger than 75 years”. In short, it is a close call; there are potential benefits and harms, and whether you think the benefit is worth the risk is a matter of personal judgement. This is why the Taskforce, and other health agencies such as Cancer Council Australia, Andrology Australia, the UK National Screening Committee and the National Screening Unit in NZ all suggest that men should be adequately informed about the pros and cons of PSA screening before going ahead with a PSA screening test.
What is the benefit of screening for prostate cancer?
Let’s now take a careful look at what we really know about whether screening for prostate cancer saves men from dying early from prostate cancer. We will look at a “randomised clinical trial” or a “randomised controlled trial” in relation to prostate cancer screening to see how do these trials differ from other “studies” about the effectiveness of screening.
In short, “randomised clinical trials” or “randomised controlled trials” provide a much higher level of evidence than other “observational studies”. 95
Many research reports published in the medical literature are what are known as “observational studies”. An example of a basic observational study would be when a group of people who smoke are followed for a long time, maybe 20 years, to see how many people develop lung cancer, heart disease and so on. Their results are compared with a control group of non-smokers who are also followed for the same time. The temptation is to see any differences in the disease patterns of the two groups as being attributable to (here) the smoking and nothing else. While these studies are sometimes very important they have a big weakness, which is that you can never be sure that the exposed group (in this example the smokers) aren’t different in some important way from the control group (in this example the non-smokers). For example, the non-smokers may be healthier in other ways such as exercising more or eating better, and it could be these differences that are important rather than the smoking itself (although with this example, the weight of evidence is overwhelming that smoking is so risky that it overwhelms all other considerations).
We now have many examples of how we have been misled by relying on these kinds of observational studies. A recent example was hormone therapy (HT) and heart disease. On the basis of observational studies, it was long believed that HT should lower the risk of heart disease in post-menopausal women and on this basis many women around the world were prescribed HT for many years for this and other possible benefits. However when the big randomised controlled trials were finally done, it was clear that HT does not prevent heart disease in these women and may even increase the risk. In short, we learnt the hard way that relying on observational studies to decide what works in health care isn’t good enough. And this is particularly true of screening when it’s very easy to be misled by observational studies as there can be many, and subtle, differences between screened and non-screened people. 96
So instead we rely on randomised controlled trials (RCTs) in prostate cancer screening. These provide much stronger evidence because we compare two groups of people – men who have been allocated at random (in a process like a lottery) to be screened with men who have been allocated at random to no screening. This means there shouldn’t be any important differences between the groups we are comparing other than participation in prostate cancer screening.
With an RCT examining the power of PSA testing to save lives, men in the at-risk age group (over 50) who have not had a PSA test are randomly allocated either to the “intervention” group (i.e. they will be asked to have a PSA test every year or few years) or to the “control” group (i.e. they are not given a PSA test). The men in both groups are then followed by researchers over a number of years to see what happens to them. Here, the main outcomes of interest are simply “what proportion of men in the intervention and control groups develop prostate cancer, and what proportion die from it?”
When it comes to RCTs examining the impact of PSA screening on death from prostate cancer, the first challenge is to find large populations of men who have not already had a PSA test. In the USA, the promotion and uptake of PSA screening has been so large that a recent long-awaited RCT examining whether prostate screening saves lives was badly affected by many of those who were assigned to the “no screening group” in fact getting screened. “Rates of screening in the control group increased from 40% in the first year to 52% in the sixth year for PSA testing and ranged from 41 to 46% for digital rectal examination” [105]. This “corruption” of the control group badly affected the ability of the study to test whether screening made any difference in preventing death. The published results of the US trial showed no survival benefit from screening but because of this trial “corruption”, that study provides little insight into whether screening “works”. 97
Does screening for prostate cancer save lives?
Our knowledge of this advanced significantly in 2009 with the publication of a major nine-year-long study, the European Randomized Study of Screening for Prostate Cancer (ERSPC) [18]. In this large RCT study, the prostate cancer death rate in men who were screened was compared with the rate in men who were not screened. If early detection were beneficial, we would expect that in the screened group, that there would be a lower rate of death from prostate cancer because many life-threatening cancers would have been detected early and the men put through treatment. If it were true that early detection and treatment of men saved lives, the rate of death from prostate cancer in the screened men should be lower than in the non-screened men.
The ERSPC [18] commenced in the early 1990s. The study included 182,000 men aged 50 to 74 years from seven European countries. Some of these men were randomly assigned to a group that was offered PSA screening at an average of once every four years (average 2.1 tests in the nine years). Others were assigned to a control group that did not receive such PSA testing. The primary outcome of interest to the study authors was the rate of death from prostate cancer.
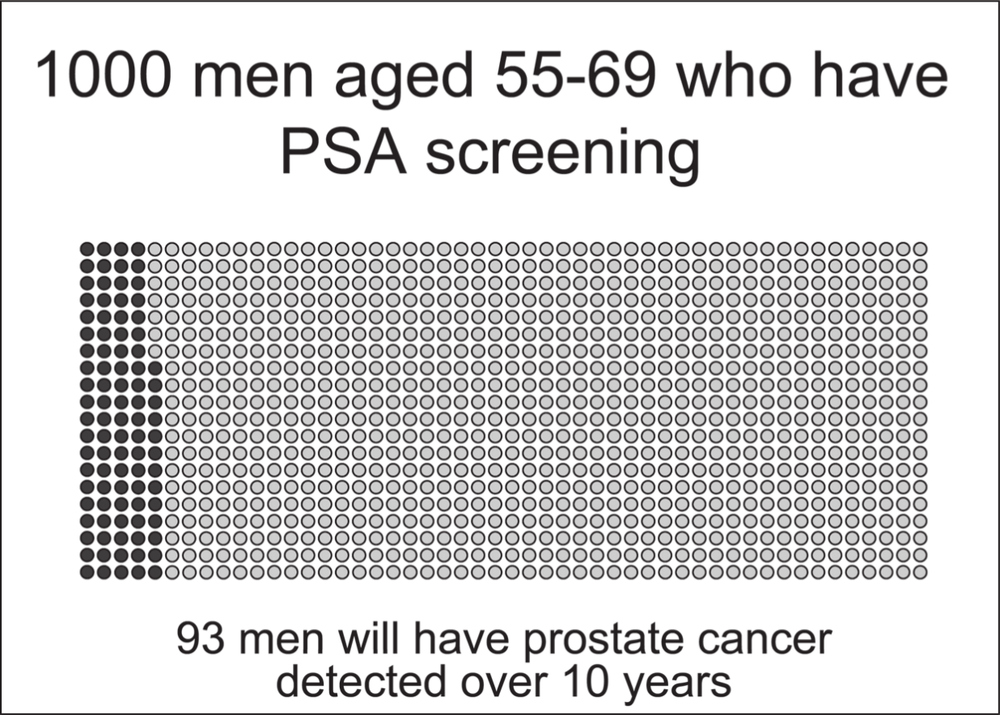
During a median follow-up of nine years, the cumulative incidence of prostate cancer diagnosed in the screened men was 82 per 1000 men and 48 per 1000 men in the control group. Basically this means that in the nine years after the men were first screened, nearly double the rate of prostate cancers was found in men who were screened at an average of once every four years than was found in the men who were not offered screening. The prostate cancers found in the non-screened control group would have been found because some of these men would have experienced symptoms and gone to see 98a doctor. Investigations would have then found prostate cancer in these men.
So far then, we can say that by screening lots of men, we will find nearly twice as many histologically (laboratory) confirmed cancers in those screened men than in men who don’t get screened but who present to doctors with symptoms which are then investigated and found to be cancer. But what we really need to know is how many men in the screened and unscreened groups died from prostate cancer in the nine years of the study, the main focus of interest.
The results were 2.94 deaths per 1000 men in nine years in the group of screened men. In the control group, there were 3.65 deaths per 1000 men in nine years. The difference means that screening prevented just 0.71 deaths per 1000 men over nine years. This is about a 20% reduction (in relative terms) in the risk of dying from prostate cancer (0.71/3.65). Now that might not sound very much, but nine years isn’t very long in the course of a slow disease like prostate cancer. Also as we can readily see, dying from prostate cancer is uncommon in men this age, so as we expect, the death rate is low in both groups. In other words it is hard to see a big effect because the outcome is relatively uncommon to start with.
This compares well with the results of randomized studies of mammographic screening for breast cancer in women. Systematic reviews of these trials conclude that among women aged 50–69 years screening with mammograms produces a relative benefit of about 15% [106].
The key issue that all men need to consider, however, is the balancing of benefits versus harms. So the ERSPC study found that we can reduce the risk of dying from prostate cancer from 3.65 deaths per 1000 men over nine years to 2.94 deaths per 1000 men over nine years. The price of this modest benefit is the extra numbers of men diagnosed with and treated for prostate cancer. Instead of having 48 99per 1000 men affected by a prostate cancer diagnosis and treatment, as in the control group, there were 82 men affected in the screened group, in order to prevent less than one death per 1000 men. Whether you think that is a reasonable price to pay depends on how you feel about the psychological and physical side effects of having a prostate cancer diagnosis and treatment (more on that below).
The investigators of the study used these numbers to calculate that 1410 men would need to be screened and 48 additional cases of prostate cancer would need to be treated to prevent a single death from prostate cancer. For some readers, this might be a bit hard to follow. Put another way, suppose these 48 men were to gather in one room. Each of them would be convinced that the detection and treatment of their prostate cancer had saved their life. And 47 of the 48 would be wrong.
The following may also help. The New York Times ran a report on PSA screening on 19 March 2009 [107] describing the study as “the first based on rigorous randomized trials”. In summarising the results, the NYT quoted Dr Peter Bach, a physician and epidemiologist at Memorial Sloan-Kettering Cancer Center. Bach suggested that one way to think of the results of the European trial was to consider a man having a PSA test that needed further investigation:
It leads to a biopsy that reveals he has prostate cancer and he is treated for it. There is a one in 50 chance that in 2019 or later he will be spared death from a cancer that would otherwise have killed him. And there is a 49 in 50 chance that he will have been treated unnecessarily for a cancer that was never a threat to his life.
Before we leave this study, some other results from it were that in the screening group, 82% of men accepted at least one offer of screening. During the trial, 126,462 PSA-based tests were performed 100on men in the screening group. In total, 16.2% of these tests were positive and 85.8% of the men with positive PSA results took up the recommendation to have a biopsy. Of the men who underwent biopsy, 75.9% had a false positive result (in other words, their elevated PSA did not translate to laboratory confirmed prostate cancer). The proportions of men who had a Gleason score of 6 or less were 72.2% in the screening group and 54.8% in the control group, and the proportions with a Gleason score of 7 or more were 27.8% in the screening group and 45.2% in the control group. This is to be expected because screening detects cancers earlier, and also, as we have seen, finds many low grade cancers.
While we have tried to explain the complexities of the European trial carefully to maximise its comprehensiveness to men without epidemiological training, we appreciate that for some its meaning will still be unclear. Over the next pages we present the “take-home” messages in graphic form via diagrams with a single dot representing one of a 1000 men. We hope this information will assist in your understanding. 101

 102
102

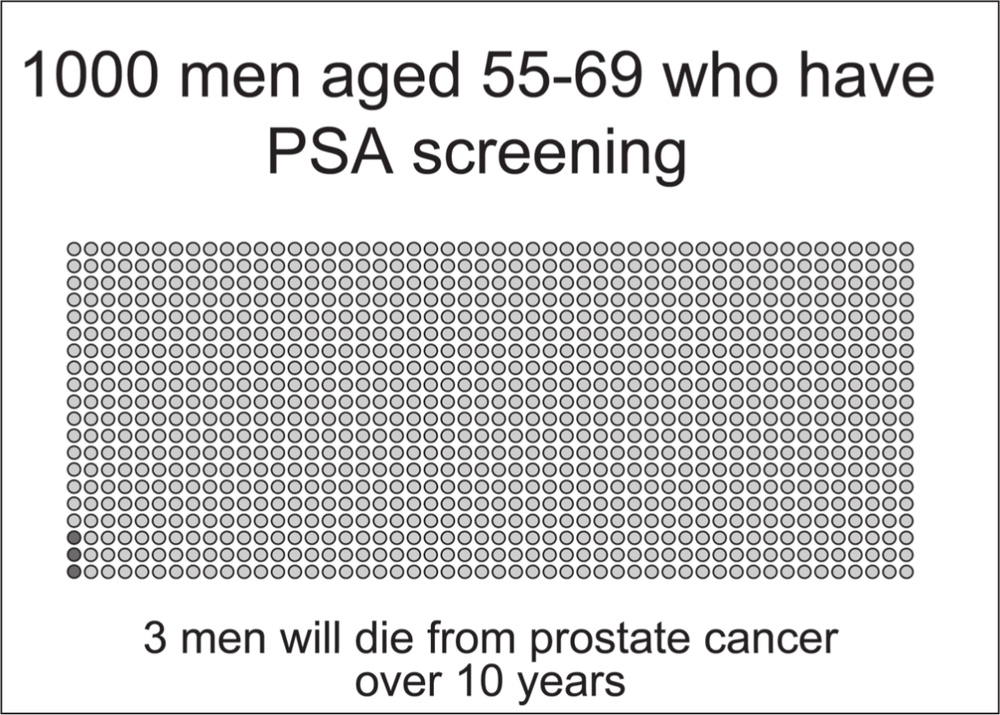
Source: Data from the European Randomized Study of Screening for Prostate Cancer; illustrations by Erin Mathieu, Sydney School of Public Health, University of Sydney103
The 2010 Swedish Göteborg study
The Göteborg (Sweden) trial was a randomised controlled trial in which men aged 50–64 were randomly allocated to no screening or PSA screening every two years. After 14 years of follow-up, the study found that PSA screening reduced the men’s chances of dying of prostate cancer by nearly half. Over 14 years, 0.5% of the men in the screened group (that is 0.5 per 100 men, or one man in every 200 men) died from prostate cancer compared with 0.9% of the men in the control group who were not screened (0.9 per 100 men or just under one in every 100 men). Being screened also (unsurprisingly) increased the men’s chances of having prostate cancer diagnosed; over 14 years, 12.7% of the men in the screened group were diagnosed with prostate cancer compared with 8.2% of the men in the control group. Treatments for screening detected cancers included radical prostatectomy (about 40%), radiation therapy (8%), hormone therapy (7%), surveillance followed by treatment (15%) and surveillance only (about 30%).
Expressed another way, the results of this study were that compared to a situation of no screening, 293 men need to be invited for PSA screening and 12 additional men will be diagnosed with (and treated for) prostate cancer to prevent one death from prostate cancer over 14 years.
These results are considerably better than those obtained in the European multi-nation trial. So which study is more important in the Australian debate? Which “take-home” message is most important for Australian men to consider? A commentary [33] published in Lancet Oncology in the same issue as the trial results sought to answer the question “why are there these differences between ERSPC and Göteborg?” The University of Cambridge’s David Neal addressed this important question this way: 104
Probably the most important points are the longer length of time since randomisation and the younger age at screening than in the ERSPC, in a national context of a low baseline rate of PSA testing before the study … The [Göteborg] study by Hugosson and colleagues might be generalisable to populations that have not had prior extensive PSA testing, but probably not generalisable to populations that have had such testing – eg, in the Göteborg study only 56% of cancers were low-risk according to the D’Amico criteria, by contrast with tumours found in the second or additional rounds of screening in the ERSPC, and particularly with tumours found in the course of PSA testing in the USA, where typically low-risk cancers would be found in 75% of patients.
This point was also made in a commentary published by the US National Cancer Institute:
During the course of the trial, the state of prostate cancer screening in Sweden was “very different from the situation in the United States right now,” explained Dr. Eric Klein, chair of the Glickman Urological and Kidney Institute at the Cleveland Clinic. “It’s comparable to when PSA was introduced in the United States in the late ’80s. Now we have a heavily screened population, which is why it makes sense to build on the results of this trial to further refine our screening efforts to identify men at risk for potentially lethal cancers.” (see www.cancer.gov/ncicancerbulletin/071310/page5)
Like the USA, Australia is a nation which has had extensive “de facto” screening of the population for at least 10 years with more than 50% of men having been tested at least once [16]. This means that the Australian population, many of whom have already been tested, might be expected to show less benefit of screening than was found in Goteborg. 105
What are the harmful effects of screening for prostate cancer?
Overdiagnosis: cancer treatments you didn’t need
As we have emphasised throughout the book, there is a big risk with PSA testing of dredging up cancers that would have remained silent and not caused symptoms throughout life. These cancers would only be found if the person happened to have an autopsy after they died (or if they were screened for prostate cancer).
These cancers are called overdiagnosed cancers. Unfortunately our tests are not yet good enough to distinguish between overdiagnosed cancers and symptom-causing, life-threatening cancers. So we offer treatment to everyone with prostate cancer. These cancer treatments commonly have adverse effects and sometimes those adverse effects can be really bad for a person’s quality of life; they can be long lasting or even life threatening. This makes overdiagnosis and consequent overtreatment the number one harmful effect of prostate cancer screening.
The US Preventive Health Task Force’s 2008 review of the evidence [13] on prostate screening concluded that
Modeling studies based on U.S. incidence data suggest overdiagnosis rates ranging from 29% to 44% of all prostate cancer cases detected by PSA screening [108]. Because patients with ‘pseudo-disease’ receive no benefit from, and may be harmed by, prostate cancer screening and treatment, prostate cancer detection in this population constitutes an important burden.
Thanks to the results from the ERSPC nine-year trial of screening [18], we know that about one in 48 men with screen-detected prostate cancer will have death from prostate cancer prevented by screening (see above). This means the other 47 men have prostate cancer which 106is overdiagnosed and overtreated, in the sense that it would not have killed them had it not been found and not treated. Recall however, that the follow-up time of the European trial was only nine years. It is possible that had the trial gone for longer (say 20 or 30 years) more of these 48 men may have benefited and had a prostate cancer death averted. This seems very plausible because as we saw earlier, prostate cancer deaths increase with age. On the other hand it’s possible the number of prostate cancer deaths prevented might not have got any greater had the trial gone for longer. We just don’t know.
As we saw, the Göteborg study published data on the number of men who could be expected to benefit from screening, that is, prevent death from prostate cancer. They estimated that about one in 12 men with screen-detected prostate cancer will have death from prostate cancer prevented by screening within a time frame of 14 years. This means only 11 men would have been overdiagnosed and overtreated prostate cancer. This better result is likely due to a combination of factors including the longer length of follow-up time (14 years rather than nine years) and the difference in study population (that is men who were largely unscreened for prostate cancer). It is likely that the numbers for Australian men would lie somewhere between these two estimates.
What we do know is that, regardless of which study you elect to put your faith in, the vast majority of men who have prostate cancer found by PSA testing do not benefit, or in other words, do not have a prostate cancer death prevented. It’s also pretty clear that if you have screening-detected prostate cancer found and treated you are much, much more likely to be experiencing overdiagnosis and overtreatment than you are to be having a prostate cancer death prevented. 107
False alarms
While overdiagnosis may be the biggest downside of PSA screening, false alarms are a considerable problem too. In the European study, 16% of PSA tests were abnormal leading men to have a biopsy. About a quarter of these men were found to have prostate cancer on their follow-up biopsy. In other words, about 75% (three quarters) of men who had a biopsy triggered by a raised PSA test result experienced a false alarm [18]. This means they had an abnormal PSA test result but their subsequent prostate biopsy showed no cancer. There are both psychological and physical downsides of having a false alarm. Some people describe it as the scariest time of their lives. For most, it is at least inconvenient, uncomfortable and anxiety provoking to some extent [109]. While most people (more than 90%) do not experience any important physical adverse effects of the biopsy, a few people (less than 1%) suffer important complications particularly infection, which can be serious enough to require intravenous antibiotics and hospitalisation [87].
It is important to remember that the chance of experiencing a false alarm increases as you have more screening tests. So while the chance of having a false alarm is in the range 3–10% following one PSA test, if you have tests on a regular basis (say yearly or every two years) the chance of having a false alarm after one of them becomes quite high over a “lifetime” of screening.
Make your own choice: weighing up the benefits and harmful effects of prostate cancer
As mentioned earlier, there is considerable interest in providing men with decision tools to help them weigh up the benefit versus risks of PSA screening. A number of these “decision aids” have been developed and tested in the US, Canada and the UK. One such decision aid, Prosdex, was developed and evaluated in Wales. It is available for free online at www.prosdex.com